Pharmaceutical Sciences. 2025;31(3):279-287.
doi: 10.34172/PS.025.42098
Research Article
Anti-stress Effects of Chrysin on Rats: A Metabolomics Study
khadijeh Haghighat Data curation, Writing – original draft, 1 
Fariba Mahmoudi Conceptualization, Data curation, Writing – original draft, 1
Maryam Khoshkam Conceptualization, Methodology, Supervision, 2, 3, * 
Homayoun Khazali Writing – review & editing, 4
Author information:
1Department of Biology, Faculty of Sciences, University of Mohaghegh Ardabili, Ardabil, Iran
2Department of Chemistry, Faculty of Sciences, University of Mohaghegh Ardabili, 56199-11367, Ardabil, Iran
3Department of Chemistry, Faculty of Sciences, University of Zanjan, 45371-38791 Zanjan, Iran
4Department of Animal Sciences and Marine Biology, Faculty of Life Sciences and Biotechnology, Shahid Beheshti University, Tehran, Iran
Abstract
Background:
Chrysin is a phytochemical compound and was found to be a potent anti-anxiety and neuroprotective. However, it is unclear whether chrysin has beneficial effects on stress-related metabolomic profiles. The current study aimed to assess the anti-stress effects and corresponding mechanisms of chrysin on male rats using a metabolomics method.
Methods:
The male rats weighing 220±10 g segregated into different groups (n=6). To induce stress, animals subjected to stress for 2 hours. The control and stressed groups received saline. Also, the intact and stressed groups, received chrysin at doses of 10 mg/kg intraperitoneally (IP). Then, behavioral tests were performed. All injections performed 30 min before stress induction. Then the serum was collected. The metabolic profiles were analyzed using the HNMR method.
Results:
Serum metabolic profiling showed comprehensive metabolic variation among the four groups. A series of metabolic pathways including pyrimidine, arginine and phenylalanine-tyrosine-tryptophan biosynthesis were affected. Eighteen potential biomarkers such as phenylalanine, tyrosine, alanine and arginine were identified. Chrysin could correct the disturbed metabolic pathways and restore the variation of these potential markers (P≤0.05). Also, behavioral tests showed a significant improvement in anxiogenic behaviors in the male rats receiving chrysin compared to the stress group (P≤0.05).
Conclusion:
The metabolic changes and the associated pathways, provide insights into the mechanisms of anti-anxiety of chrysin, and further studies are needed to confirm its anti-anxiety effect.
Keywords: Chrysin, Metabolic profiles, Phytochemical, Rat, Stress
Copyright and License Information
© 2025 The Author(s).
This is an open access article and applies the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This research was funded by University of Mohaghegh Ardabili (The article is extracted from the student’s dissertation).
Introduction
Stress is defined by physiological alterations in response to any threatening stimuli. Depression and anxiety disorders are the consequences of stress. The World Health Organization lists anxiety and stress disorders as one of the top ten public health concerns, which have become epidemic in recent years. These conditions are known to be significant risk factors for various illnesses, such as metabolic, neuropsychiatric, and cardiovascular. Currently, treatment options include anti-anxiety medications such as beta blockers, antidepressants, and members of the benzodiazepine class. However, there are various issues, such as tolerance building, withdrawal symptoms, low success rates, and adverse effects of drug therapy.1,2 Therefore, the disadvantages of conventional anti-anxiety drug have prompted the search for alternative treatments such as bioactive compounds of herbal medicines and their derivatives.2
Chrysin (5,7-dihydroxyflavone) is one of the main biologically active compounds of plants including Passiflora, Scutellaria baicalensis, propolis and honey.3 Previous studies, have been demonstrated the antioxidant, anti-inflammatory, neuroprotective, anti-stress, and analgesic effects of chrysin.4 It has been established that chrysin exerts anxiolytic effects by activating the GABAergic signaling system.5 Furthermore, the evidence shows that chrysin, could act as a positive moderator on behavioral changes in stressed rat.6 Several studies have been conducted on the toxicity of chrysin in animal models. Chrysin is well tolerated at low doses and does not cause significant side effects. At higher doses, side effects are mild and transient. Also, no toxicity has been reported after volunteers consumed oral doses of chrysin ranging from 300 to 625 mg.7 Some studies also show that chrysin does not negatively affect the function of organelles such as the liver and kidneys and may even help improve their function.8
Few studies have used serum metabolomics techniques to examine the pharmacological mechanisms of chrysin. Metabolomics is a powerful approach to investigate all of the endogenous metabolites that biological systems produce. The metabolomics method makes use of bio-fluids, which are easily obtained without causing any harm, and include a large range of metabolites. This approach has been extensively used in recent years to investigate the processes and effects of drugs.9,10 The present study investigated changes in metabolic profiles and stress-related behaviors in a male rat stress model treated with chrysin.
Methods
Animals
The animals maintained in order to compatibility in the laboratory for two weeks. Male rats had free access to food and water. The laboratory temperature was set at 22-25 °C under 12 hours of alternating light and dark cycles.
Design and treatment
To conduct the experiment, 24 male Wistar rats weighing 220 ± 10 g divided into four groups (n = 6). Group I: received saline as a control group. Group II: received saline as a stressed group. Groups III and IV: received 10 mg/kg chrysin (Cas No, 480-40-0, Co, USA), as a stressed and intact groups. The drugs were injected intraperitoneally (IP). All injections performed 30 min before stress induction. Animals euthanized at the end of the experiment. Then, blood samples were collected.
Acute restraint stress
For acute stress induction, 30 minutes after receiving chrysin, animals placed in a restraining box with dimensions (length 18 cm, diameter 5 cm). Then, the boxes were moved to a quiet, dark room for 2 hours.11 All experiments were done between 9-11 AM.
Behavioral tests
Open field test (OFT): This test is used to assess the stress in male rats. The animals were placed in the middle of a box (60 × 60 × 60 cm) divided into 16 equal squares. Then, the number of enteries into center and the time spent in center by male rats were evaluated for 5 minutes.12
Forced swimming test (FST): For this test, a transparent container 30 cm long, 30 cm wide, and 60 cm high was used. Two-thirds of the container was filled with water and the temperature was set at 23-25 °C. One day before the experiment, the animals were placed in the swimming container for learn to swim. To perform the test, the male rats were slowly placed in water. Then, the anxiogenic behaviors were recorded by the camera for 6 minutes. Active movement of arms and legs indicates swimming.12
Light/ dark test (LDT): The box consists of two light (40 × 40 × 60 cm) and dark (20 × 40 × 60 cm) compartments. These two compartments are connected by a valve (10 × 10 cm). To do the test, first, a male rat is placed in a dark compartment. After 10 secs, the valve is opened, so that the animal can move freely between the two compartments. The number of entries into the light compartment and the time spent in the light compartment were recorded for 5 minutes by the camera.6
Serum preparation for 1HNMR analysis
The collected blood samples were centrifuged at 3000 rpm for 15 minutes. The isolated serum samples were stored at – 80 °C, immediately for further analysis by 1HNMR spectroscopy. Then, 300 μL of prepared serum diluted in 100 mL D2O. 1HNMR spectra of serum samples were recorded using a 500 MHz Varian Unity Inova NMR at 298 °K using a 5 mm probe. The standard Carr-Purcell-Meiboom-Gill (CPMG) pulse was applied using the water suppression and a weak irradiating pulse on the water peak during the saturation delay. To obtain the spectra, 100 scans were performed into 32 k data points. A relaxation delay of 2.0 s and an acquisition time of 3.27 s were assumed for the 1HNMR spectrum. TMS was used as an internal reference.
Preprocessing of 1HNMR spectra and statistical analysis
After recording spectra, the FID spectra were converted to frequency domain spectra using MestRenova (Mestrelab Research Mnova 15.0.0.34764) software. The spectra were phase and baseline corrected and water suppression was done at 4.7-5.52 ppm in data. The spectra were imported to MATLAB R2022a (MathWorks) for further analysis. The imported data was an 18 × 32 768 matrix in which, 24 was the number of serum samples and 32 768 was the number of chemical shifts. In the next step, the unwanted regions including only the noise in spectra were removed from first and end of spectra and then the data were aligned using correlation optimized warming COW algorithm. In the next step, data were binned to reduce the size of data. After binning the data size was 18 × 6596. Then the data were scaled with pareto scaling.13 After scaling the pre-processed data was ready to multivariate analysis. In the first step Principal Component Analysis (PCA) was performed on data. In the next step, Partial Least Square-Discriminant Analysis (PLS-DA) as a supervised classification model was applied on data. To find the altered metabolites, variable important predictor (VIP)score from PLS-DA was calculated and the important chemical shifts (VIP > 1) was used to estimate important altered metabolites. The metabolites were found from www.hmdb.ca database.14 After finding the important metabolites, these metabolites were entered to MetaboAnalyst 5.0 to identify altered metabolic pathways.15
The data were analyzed using SPSS software (version 16) and one-way ANOVA. Tukey’s test was used to show differences between groups. The level of statistical significance was considered as P values ≤ 0.05. The results were expressed as a mean ± SEM.
Results
The effect of chrysin on anxiogenic behaviors
As shown in Figure 1A and 1B, the time spent into the center and the number of entries into center of the open field box in the stress group were significantly decreased compared to the control (P ≤ 0.05). In the stressed group receiving chrysin 10 mg/kg, the time spent into the center and the number of entries into center increased significantly compared to the stress group (P ≤ 0.05). In the intact group receiving chrysin 10 mg/kg, compared to the control, the time spent into the center and the number of entries into center decreased significantly (P ≤ 0.05).
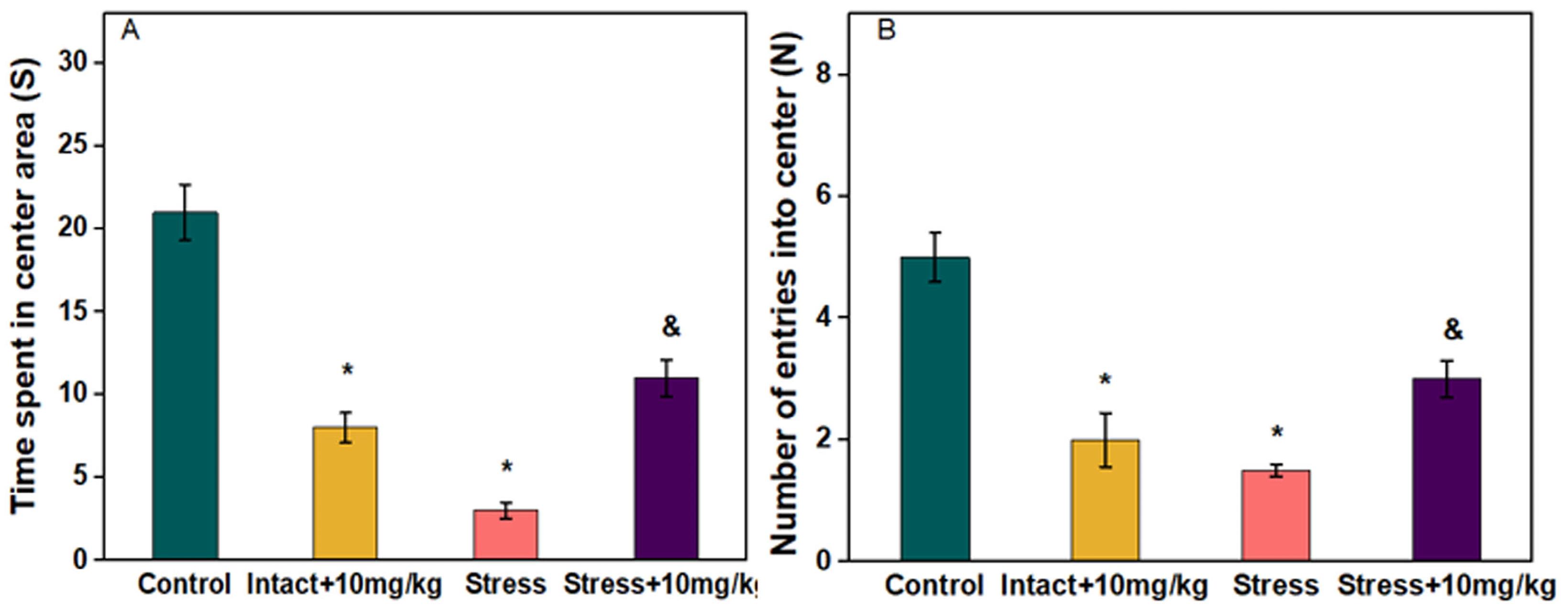
Figure 1.
Effect of chrysin administration on (A) time spent in center and (B) number of entries into center in OFT. Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test. All values are presented as means ± SEM. (P ≤ 0.05) *: compared with control, &: compared with stress group (n = 6)
.
Effect of chrysin administration on (A) time spent in center and (B) number of entries into center in OFT. Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test. All values are presented as means ± SEM. (P ≤ 0.05) *: compared with control, &: compared with stress group (n = 6)
The results of the light/dark box showed that the time spent in the light compartment was not significantly reduced in the stress group compared to the control. Injection of chrysin 10 mg/kg into stressed groups did not significantly increase the time spent in the light compartment compared to the stress group (Figure 2A). Also, in the intact group receiving 10 mg/kg chrysin, the time spent in the light compartment did not decrease significantly compared to the control.
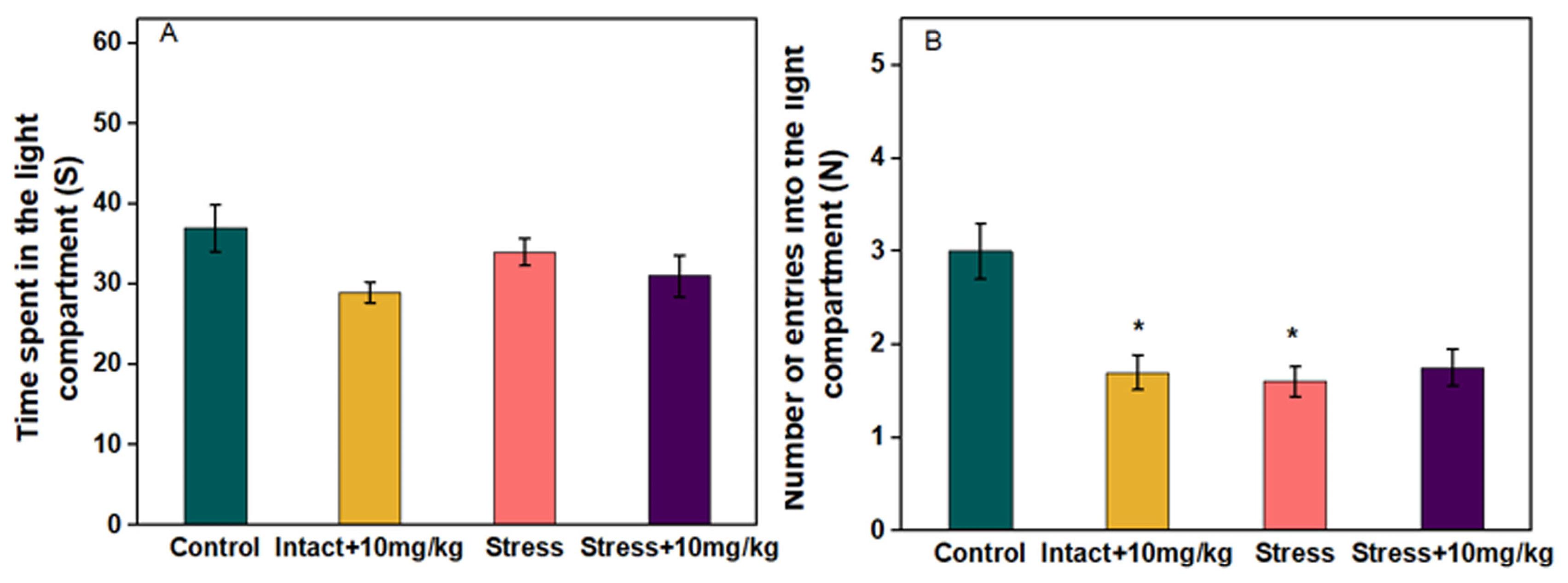
Figure 2.
Effect of chrysin administration on (A) time spent in the light compartment and (B) number of entries into the light compartment in LDT. Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test. All values are presented as means ± SEM. (P ≤ 0.05) *: compared with control, &: compared with stress group (n = 6)
.
Effect of chrysin administration on (A) time spent in the light compartment and (B) number of entries into the light compartment in LDT. Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test. All values are presented as means ± SEM. (P ≤ 0.05) *: compared with control, &: compared with stress group (n = 6)
The stressed group compared to the control showed a decrease in the number of entering into the light compartment. The decrease was significant (P ≤ 0.05). In the stressed groups receiving chrysin 10 mg/kg compared to the stress group, the number of entering into the light compartment increased. The increase was not significant (Figure 2B). The number of entering into the light compartment in the intact group receiving chrysin 10 mg/kg was significantly reduced compared to the control (P ≤ 0.05).
The results of FTS showed that the increase in the duration of immobility in the stress group compared to the control was significant(P ≤ 0.05). Injection chrysin 10 mg/kg into stressed male rats significantly decreased the duration of immobility compared to the stress group. (P ≤ 0.05) (Figure 3). The duration of immobility in the intact group receiving 10 mg/kg of chrysin did not increase significantly compared to the control.
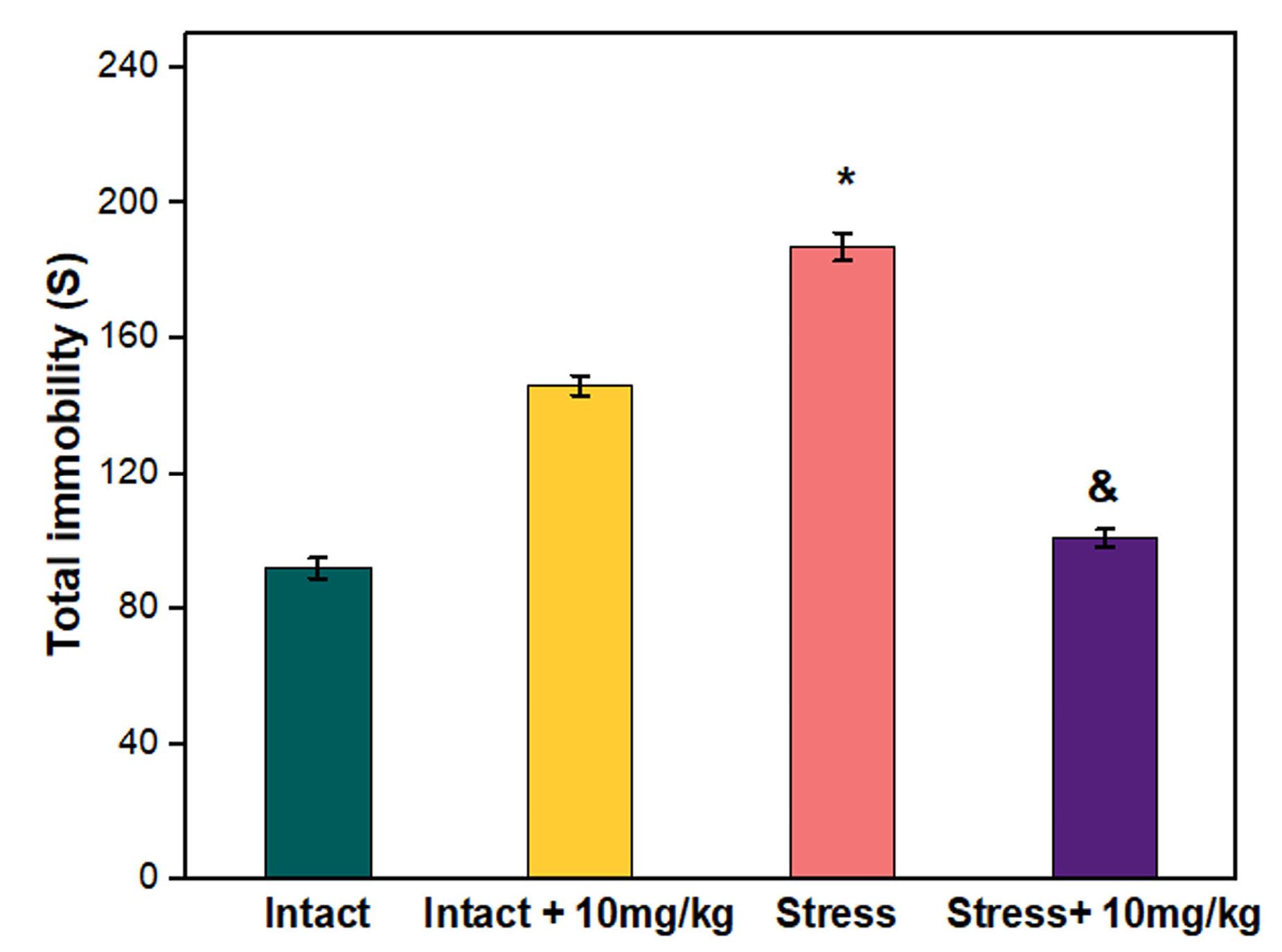
Figure 3.
Effects of chrysin administration on immobility time in FST. Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test. All values are presented as means ± SEM. (P ≤ 0.05) *: compared with control, &: compared with stress group (n = 6)
.
Effects of chrysin administration on immobility time in FST. Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test. All values are presented as means ± SEM. (P ≤ 0.05) *: compared with control, &: compared with stress group (n = 6)
The effect of chrysin on profile metabolic changes
Figures 4a and 4b show the resulted score and loading plot of data (PCA). From this figure it is clear that the groups are not separated from each other. Figures 4c and 4d show the score and loading plots from PLS-DA. From figure, a good separation between groups is observed. The scores plot showed the treated groups with chrysin were far from the control group, which showed positively correlated with the score plots. Chemical shifts with VIP > 1 were selected to estimate the changed metabolites. To examine if chrysin treatment changed metabolites in stress conditions, we found potential biomarkers, which were significantly different between stress + chrysin treatment group, chrysin + intact group, stress group and control group in the serum samples, and are summarized in Table 1.
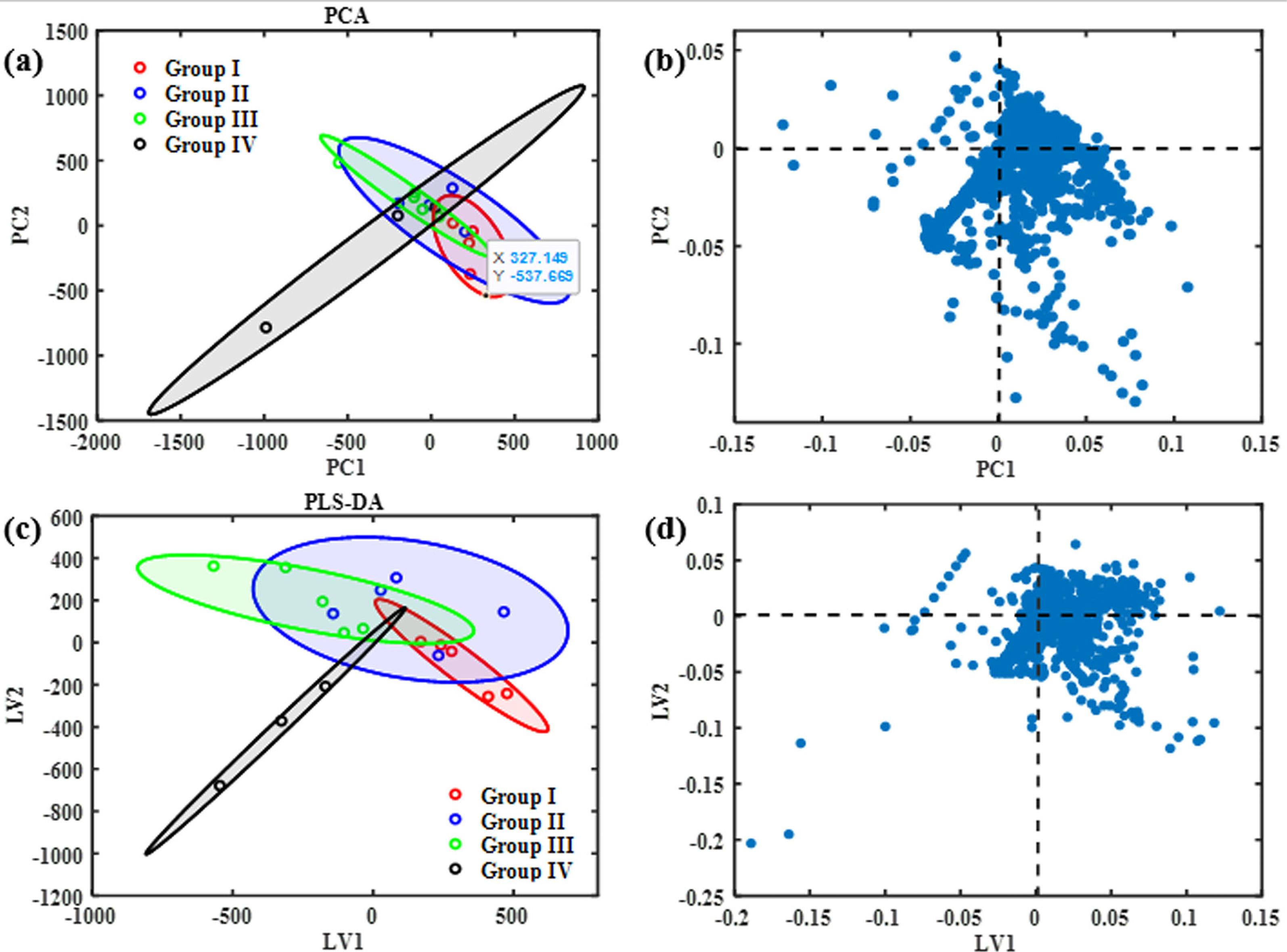
Figure 4.
Estimated (a) score with 95% confidence ellipse and (b) loading plot from applying PCA on data and (c) score plot and (d) loading plot from PLS-DA on data. Group I: received saline as a control. Group II: received saline as a stress group. Group III: received 10 mg/kg chrysin, as stressed rats, Group IV: received 10 mg/kg chrysin, as intact rats. Data were analyzed using MestRenova (Mestrelab Mnova VBuild 18998) software and MATLAB R2022a (MathWorks). PC: Principal component, LV: Latent variable
.
Estimated (a) score with 95% confidence ellipse and (b) loading plot from applying PCA on data and (c) score plot and (d) loading plot from PLS-DA on data. Group I: received saline as a control. Group II: received saline as a stress group. Group III: received 10 mg/kg chrysin, as stressed rats, Group IV: received 10 mg/kg chrysin, as intact rats. Data were analyzed using MestRenova (Mestrelab Mnova VBuild 18998) software and MATLAB R2022a (MathWorks). PC: Principal component, LV: Latent variable
Table 1.
Important metabolites and their table of assignment in comparison to control group
|
Metabolite’s name
|
*G21
|
*G31
|
*G41
|
| L-phenylalanine |
↓ |
↓ |
↑ |
| L-tyrosine |
↓ |
↓ |
↑ |
| Glutamate |
↓ |
↓ |
↑ |
| L-glutamine |
↓ |
↓ |
↑ |
| L-citrulline |
↓ |
↓ |
↑ |
| L-argininosuccinate |
↓ |
↓ |
↑ |
| L-Arginine |
↓ |
↓ |
↑ |
| L-ornithine |
↓ |
↓ |
↑ |
| L-glutamine |
↓ |
↓ |
↑ |
| N-carbamoylaspartate |
↓ |
↓ |
↑ |
| Cytidine |
↓ |
↓ |
↑ |
| Uridine |
↓ |
↓ |
↑ |
| Deoxycytidine |
↓ |
↓ |
↑ |
| Deoxyuridine |
↓ |
↓ |
↑ |
| Dihydrouracil |
↓ |
↓ |
↑ |
| Ureidopropionate |
↓ |
↓ |
↑ |
| B-alanine |
↓ |
↓ |
↑ |
| Thymidine |
↓ |
↓ |
↑ |
*G21: comparison of Group 2(stress group) with group 1 (control).
*G31: comparison of Group 3 (stress receiving chrysin) with group 1 (control).
*G41: comparison of Group 4 (intact receiving chrysin) with group 1 (control).
Table 2 shows the altered pathways between stress + chrysin treatment, chrysin + intact groups, stress group and control based on the information obtained from the MetaboAnalyst database. Metabolic analysis revealed that 22 metabolic pathways were involved during stress. In this study, three important metabolic pathways including phenylalanine-tyrosine–tryptophan biosynthesis, pyrimidine and arginine were selected, which had a strong effect on the metabolism of male rats during stress (Figures 5 and 6). Other pathways are not shown.
Table 2.
Results of pathway analysis using MetaboAnalyst for the stress group after treatment with chrysin
|
Metabolic profiles
|
Total
|
Expected
|
Hits
|
Raw
P
values
|
Holm adjust
|
FDR
|
Impact
|
| Aminoacyl-tRNA biosynthesis |
48 |
3.57 |
16 |
9.27E-08 |
7.78E-06 |
7.78E-06 |
0 |
| Valine, leucine and isoleucine biosynthesis |
8 |
0.59 |
6 |
3.69E-06 |
3.06E-04 |
1.55E-04 |
0 |
| Amino sugar and nucleotide sugar metabolism |
37 |
2.75 |
11 |
3.89E-05 |
3.19E-03 |
1.089E-03 |
0.321 |
| beta-Alanine metabolism |
21 |
1.56 |
8 |
6.57E-05 |
5.32E-03 |
1.38E-03 |
0.615 |
| Arginine biosynthesis |
14 |
1.04 |
6 |
2.72E-04 |
0.021 |
4.57E-03 |
0.598 |
| Pyrimidine metabolism |
39 |
2.90 |
10 |
3.51E-04 |
0.027 |
4.907E-03 |
0.209 |
| Galactose metabolism |
27 |
2.01 |
8 |
4.90E-04 |
0.038 |
5.882E-03 |
0.167 |
| Histidine metabolism |
16 |
1.19 |
6 |
6.41E-04 |
0.049 |
6.729E-03 |
0.549 |
| Arginine and proline metabolism |
38 |
2.83 |
9 |
1.31E-03 |
0.099 |
0.0122 |
0.389 |
| Taurine and hypotaurine metabolism |
8 |
0.59 |
4 |
1.61E-03 |
0.120 |
0.013 |
0.714 |
| Pantothenate and CoA biosynthesis |
19 |
1.41 |
6 |
1.80E-03 |
0.133 |
0.013 |
0.078 |
| Alanine, aspartate and glutamate metabolism |
28 |
2.08 |
7 |
3.33E-03 |
0.243 |
0.022 |
0.332 |
| Starch and sucrose metabolism |
15 |
1.12 |
5 |
3.42E-03 |
0.246 |
0.022 |
0.330 |
| Pentose and glucuronate interconversions |
18 |
1.34 |
5 |
8.14E-03 |
0.577 |
0.048 |
0.5 |
| Cysteine and methionine metabolism |
33 |
2.45 |
7 |
8.80E-03 |
0.616 |
0.049 |
0.596 |
| Thiamine metabolism |
7 |
0.52 |
3 |
1.12E-02 |
0.775 |
0.059 |
0 |
| Tryptophan metabolism |
41 |
3.05 |
7 |
2.82E-02 |
1 |
0.139 |
0.526 |
| Phenylalanine, tyrosine and tryptophan biosynthesis |
4 |
0.30 |
2 |
2.98E-02 |
1 |
0.139 |
1 |
| Ascorbate and aldarate metabolism |
10 |
0.74 |
3 |
3.27E-02 |
1 |
0.144 |
0.5 |
| Glycine, serine and threonine metabolism |
34 |
2.53 |
6 |
3.57E-02 |
1 |
0.149 |
0.202 |
| Fructose and mannose metabolism |
18 |
1.34 |
4 |
3.93E-02 |
1 |
0.157 |
0.087 |
| Phenylalanine metabolism |
12 |
0.89 |
3 |
5.37E-02 |
1 |
0.205 |
0.357 |
Note: The ‘Total’ column shows the number of compounds in each pathway. The ‘Hit’ column is the number of our query metabolites. The raw P-values are adjusted with Holm and FDR methods. The ‘Impact’ refers to the impact of each importance of pathways from topological analyses. FAD: false discovery rate.
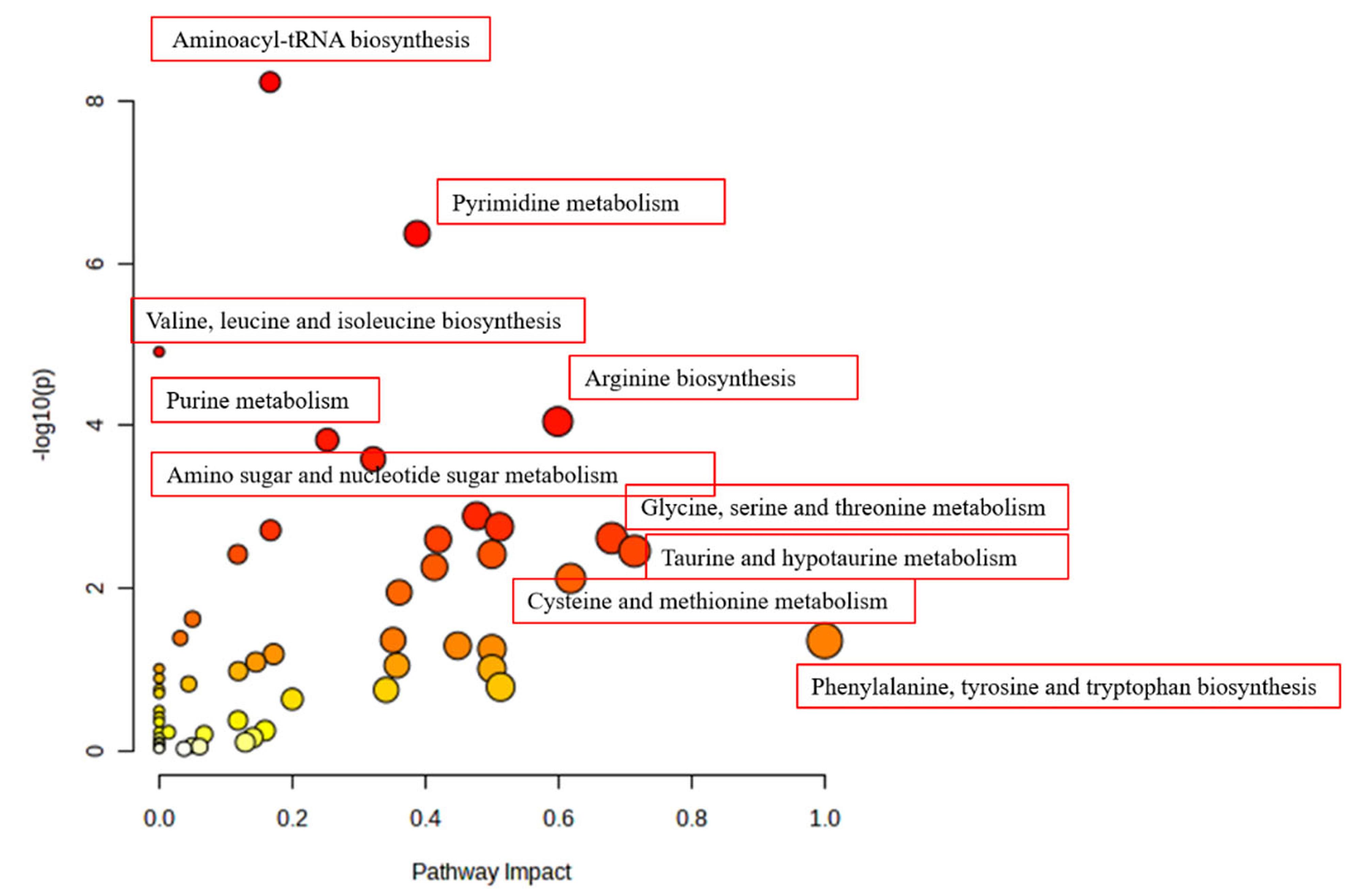
Figure 5.
Altered pathways and their impact
.
Altered pathways and their impact
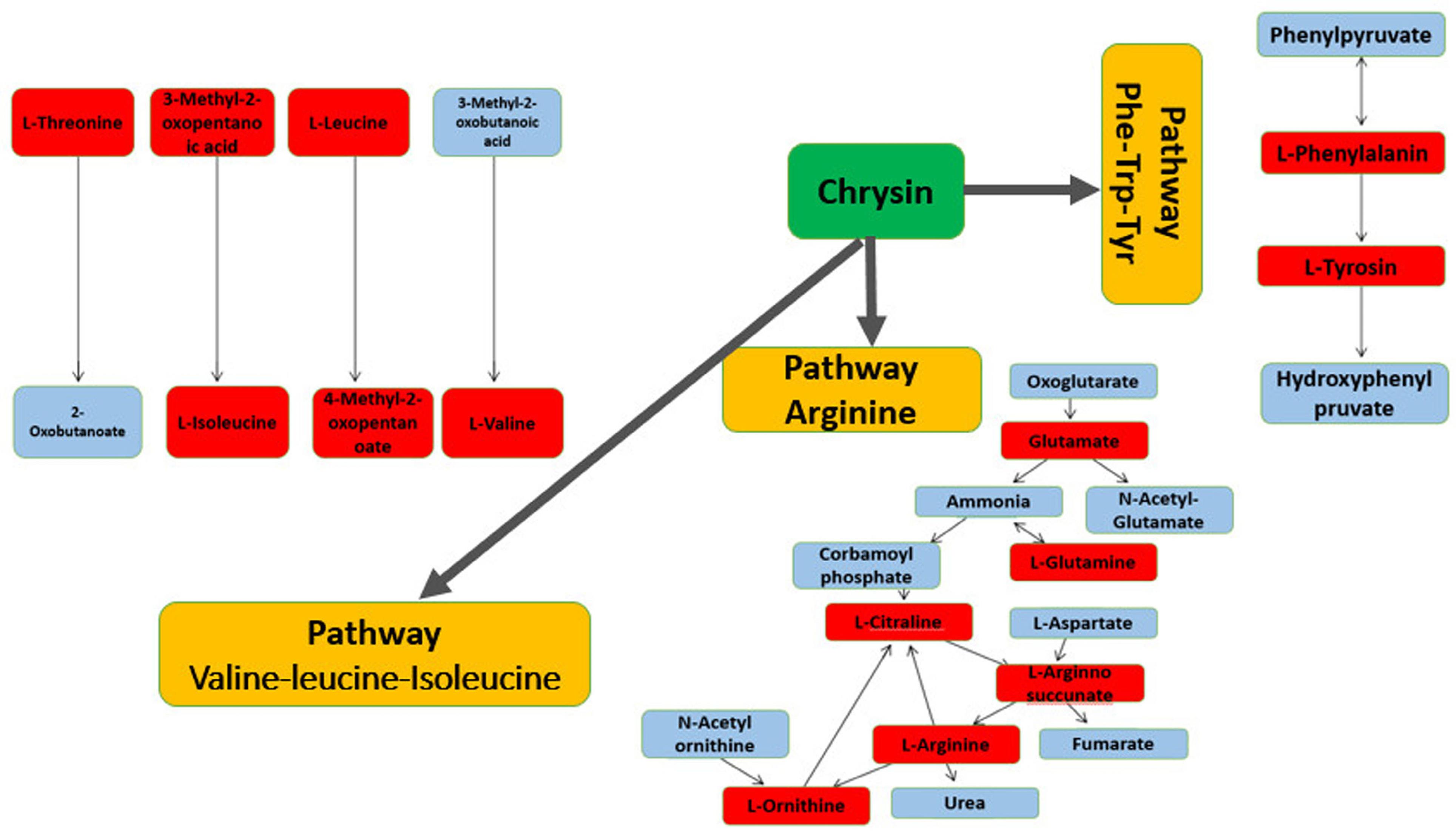
Figure 6.
Effect chrysin on biochemical pathway of pyrimidine, arginine and phenylalanine-tryptophan-tyrosine biosynthesis. The red boxes, show the altered metabolites
.
Effect chrysin on biochemical pathway of pyrimidine, arginine and phenylalanine-tryptophan-tyrosine biosynthesis. The red boxes, show the altered metabolites
Discussion
The present study showed the anti-anxiety effect of chrysin on behavior and changes in serum metabolomics profile in stress model male rats. FST, LDT, and OFT are the most commonly used tests to investigate the effects of anxiolytic drugs on behavioral parameters of animals. In the present study, acute restraint stress (2 hours) significantly reduced OFT parameters such as number of entries and the time spent in the center. Similarly, acute restraint stress significantly increased the duration of immobility in the FST, which is indicative of anxiety. The results are consistent with previous studies.16 Treatment with 10 mg/kg chrysin reversed the anxiety-related symptoms in male rats. Rashno et al showed that chrysin has anxiolytic effects,17 which coincides with our findings. One of the mechanisms involved in the response to stress is the activation of the hypothalamic-pituitary-adrenal axis (HPA). It seems that hyperactivity of the HPA axis is related to stimulating the activity of neurons containing CRH in the hypothalamus, which can be involved in the occurrence of anxiety behaviors.18 In addition, it has been reported that the GABA system is closely related to the CRH neuron, so that increasing the activity of the GABA neurons inhibits CRH secretion. According to evidence, chrysin has GABAergic effects and can exert its effect by binding the GABAA benzodiazepine site receptor.19 Therefore, there is a possibility that chrysin can create anti-anxiety behaviors with inhibiting the release of CRH via activating GABAergic system.
Anxiety disorders may lead to an increase in pro-inflammatory factors. Subsequently, the accumulation of inflammatory factors causes nerve degeneration.20,21 brain-derived neurotrophic factor (BDNF) is one of the most important molecules that plays a crucial role in the development, survival, and plasticity of neurons in the brain. BDNF is upregulated in the paraventricular nucleus of the hypothalamus in response to stress.22 Recent research has highlighted the intricate relationship between BDNF and stress, suggesting that stress can significantly influence BDNF levels by increasing pro-inflammatory factors such as tumor necrosis factor-alpha (TNF-α) and interleukins (IL-6, IL-1), that BDNF may modulate the effect of stress on the brain. Studies have shown that stress can lead to atrophy nerve, correlating with lower BDNF levels.23,21 On the other hand, antioxidant, neuroprotective and anti-inflammatory properties of chrysin have been reported. Moreover, a study shows that chrysin increases BDNF levels.24-26 Another possible mechanism of chrysin in alleviating stress can be the regeneration and protection of neurons of BDNF by reducing inflammatory factors, also evident in stress-related behaviors.
Also, in the current research, using a non-targeted metabolomics approach, eighteen metabolites with significant change levels between the chrysin treatment group, the stress group and the control group were identified and the important metabolites were investigated. Currently, no previous research has investigated the effect of chrysin on changes in metabolic profiles under stress, although there are many data on its other effects.
Phenylalanine is an essential amino acid that participates in the synthesis of proteins and catecholamines. Phenylalanine is the precursor of tyrosine and subsequently L-dopa, dopamine and neurotransmitters. A change in the metabolism of phenylalanine leads to a decrease in the level of tyrosine and its metabolites, including L-dopa, dopamine, norepinephrine and thyroxine.27 Dopamine is one of the important transmitters in the central nervous system and changes in the dopaminergic system cause many psychiatric disorders such as anxiety and depression. Studies have shown that dopamine modulates anxiety-like behavior.28 There is also a close relationship between the loss of adrenergic neurons and stress. Evidence shows that restrictive stress significantly reduced the number of noradrenergic neurons in rats, subsequently, lower levels of norepinephrine are produced.29 Tyrosine is also a precursor of thyroid hormone, which plays an important role in regulating cell metabolism, modulating neurotransmission, and neurodevelopment.27 Alterations in thyroid hormone signaling are associated with anxiety disorders. Studies show that hypothyroidism produces a mild anxiety effect in rats.30 Our results showed that the levels of phenylalanine and tyrosine decreased in the rats of the stress model compared to the control group. Previous studies support our findings.31 Injection of chrysin to intact male rats increased the level of phenylalanine and tyrosine compared to the control group. There is a possibility that in stress conditions the signaling of dopamine, thyroid hormone and norepinephrine is inefficient due to disruption in the biosynthesis of the phenylalanine-tyrosine-tryptophan pathway. Therefore, chrysin may return the condition to normal by increasing the levels of their precursors, phenylalanine and tyrosine.
Pyrimidine metabolism pathways are involved in various physiological functions. Intermediates of pyrimidine metabolism are key biomolecules in nucleic acid biosynthesis, carbohydrate and lipid metabolism.32 Previous research shows that determined aberrations in pyrimidine metabolism have been associated with neurological dysfunction and psychiatric disorders.33 In the present study, the level of pyrimidine derivatives such as beta-alanine, thymidine, glutamine and carbamoyl aspartate decreased in the stress group. This shows that targeting pyrimidine metabolism in stress model male rats is helpful in reducing anxiety. Pyrimidine bases are oxidized to beta-alanine and beta-aminoisobutyric acid. Hoffman et al, have reported that supplemented with beta-alanine leads to a decrease in anxiety in animals.34 Beta-alanine is a neurotransmitter that acts on the GABA receptor. Beta-alanine stimulates the GABA receptor.35 GABA is an inhibitory neurotransmitter that plays an important role in regulating nerve activity and reducing stress and anxiety.36 In a study, it was reported that chrysin inhibits pyrimidine biosynthesis.37 However, our findings showed that chrysin increased the levels of altered metabolites in the pyrimidine biosynthesis pathway in the intact group. Therefore, chrysin has probably led to reduced stress in animals through increasing the levels of metabolites and the effect on the GABArgic system.
L-arginine is a semi-essential amino acid, metabolized to several biologically active molecules, including L-ornithine, agmatine, nitric oxide (NO), and urea. Arginine-derived L-ornithine is converted to glutamate and glutamine.38 In the present study, the level of metabolites of ornithine, arginine, glutamate and glutamine decreased in the stress group, which can be caused by increased pro-inflammatory factors. Studies have shown that glutamine and arginine have anti-inflammatory effects so that nutrition supplements with these compounds reduces half of the cells that produce inflammatory factors (TNF-α and IL-8). An increase in pro-inflammatory factors is associated with anxiety.39 However, our results showed that, in the intact group, chrysin increased the level of metabolites of arginine, ornithine, glutamine and glutamate. However, there was a study that disagreements with our results.40 Increasing metabolites through reducing inflammatory factors can contribute to reducing anxiety. Therefore, suggests that chrysin can alleviate stress by regulating arginine pathway biosynthesis.
The present study showed that chrysin injection increased the level of metabolites of aromatic amino acids, arginine and pyrimidine in the intact group, however, in the stress group, chrysin was not able to reverse the serum levels of metabolites. Probably, the dose of chrysin was not enough to affect the stress group. Considering that, in the intact group, chrysin increased the metabolism of arginine, aromatic amino acids and pyrimidine biosynthesis pathway, which indicates the potential of chrysin in reducing stress through these pathways.
Conclusion
Briefly, we studied a novel herbal medicinal composition called chrysin on the stress. First, the effect of chrysin was investigated on the behavior of male rats. Chrysin reduced anxiogenic behaviors. Then, the effect of chrysin on metabolomics profiles was studied using HNMR- based metabolomics. It was observed that chrysin has a strong effect on pyrimidine, tyrosine-phenylalanine-tryptophan and arginine metabolic pathway compared to 22 other pathways. Chrysin increased the metabolism of these pathways in the healthy group, which indicates its positive effect. However, the effects of chrysin on metabolomic profiles were insignificant in the stress group. Therefore, one of the reasons can be the low dose of chrysin. It is suggested to work with higher doses in the subsequent studies.
Competing Interests
There is no conflict of interest in this article.
Ethical Approval
All experimental procedures were carried out in consistent with the guideline of the Ethics committee of the University of Mohaghegh Ardabili (code: IR.UMA.REC.1400.029).
Acknowledgements
The authors appreciate the University of Mohaghegh Ardabili for supplying the required apparatus.
References
- Doron R, Lotan D, Rak-Rabl A, Raskin-Ramot A, Lavi K, Rehavi M. Anxiolytic effects of a novel herbal treatment in mice models of anxiety. Life Sci 2012; 90(25-26):995-1000. doi: 10.1016/j.lfs.2012.05.014 [Crossref] [ Google Scholar]
- Sahoo S, Brijesh S. Anxiolytic activity of Coriandrum sativum seeds aqueous extract on chronic restraint stressed mice and effect on brain neurotransmitters. J Funct Foods 2020; 68:103884. doi: 10.1016/j.jff.2020.103884 [Crossref] [ Google Scholar]
- Samarghandian S, Farkhondeh T, Azimi-Nezhad M. Protective effects of chrysin against drugs and toxic agents. Dose Response 2017; 15(2):1559325817711782. doi: 10.1177/1559325817711782 [Crossref] [ Google Scholar]
- Hong JS, Feng JH, Park JS, Lee HJ, Lee JY, Lim SS. Antinociceptive effect of chrysin in diabetic neuropathy and formalin-induced pain models. Anim Cells Syst (Seoul) 2020; 24(3):143-50. doi: 10.1080/19768354.2020.1765019 [Crossref] [ Google Scholar]
- Cueto-Escobedo J, Andrade-Soto J, Lima-Maximino M, Maximino C, Hernández-López F, Rodríguez-Landa JF. Involvement of GABAergic system in the antidepressant-like effects of chrysin (5,7-dihydroxyflavone) in ovariectomized rats in the forced swim test: comparison with neurosteroids. Behav Brain Res 2020; 386:112590. doi: 10.1016/j.bbr.2020.112590 [Crossref] [ Google Scholar]
- Rodríguez-Landa JF, Guillén-Ruiz G, Hernández-López F, Cueto-Escobedo J, Rivadeneyra-Domínguez E, Bernal-Morales B. Chrysin reduces anxiety-like behavior through actions on GABA(A) receptors during metestrus-diestrus in the rat. Behav Brain Res 2021; 397:112952. doi: 10.1016/j.bbr.2020.112952 [Crossref] [ Google Scholar]
- Siddiqui A, Badruddeen Badruddeen, Akhtar J, Uddin MS, Khan MI, Khalid M. A naturally occurring flavone (chrysin): chemistry, occurrence, pharmacokinetic, toxicity, molecular targets and medicinal properties. J Biol Act Prod Nat 2018; 8(4):208-27. doi: 10.1080/22311866.2018.1498750 [Crossref] [ Google Scholar]
- Soliman MM, Aldhahrani A, Gaber A, Alsanie WF, Mohamed WA, Metwally MM. Ameliorative impacts of chrysin against gibberellic acid-induced liver and kidney damage through the regulation of antioxidants, oxidative stress, inflammatory cytokines, and apoptosis biomarkers. Toxicol Res (Camb) 2022; 11(1):235-44. doi: 10.1093/toxres/tfac003 [Crossref] [ Google Scholar]
- Ghiasvand Mohammadkhani L, Khoshkam M, Kompany-Zareh M, Amiri M, Ramazani A. Metabolomics approach to study in vivo toxicity of graphene oxide nanosheets. J Appl Toxicol 2022; 42(3):506-15. doi: 10.1002/jat.4235 [Crossref] [ Google Scholar]
- Xiong Z, Yang J, Huang Y, Zhang K, Bo Y, Lu X. Serum metabonomics study of anti-depressive effect of Xiao-Chai-Hu-Tang on rat model of chronic unpredictable mild stress. J Chromatogr B Analyt Technol Biomed Life Sci 2016; 1029-1030:28-35. doi: 10.1016/j.jchromb.2016.06.044 [Crossref] [ Google Scholar]
- Kang X, Hong W, Xie K, Tang H, Tang J, Luo S. Ginsenoside Rb1 pretreatment reverses hippocampal changes in BDNF/TrkB mRNA and protein in rats subjected to acute immobilization stress. Drug Des Devel Ther 2019; 13:2127-34. doi: 10.2147/dddt.S201135 [Crossref] [ Google Scholar]
- Pan Q, Wu J, Liu Y, Li X, Chen J. Involvement of hepatic SHIP2 and PI3K/Akt signalling in the regulation of plasma insulin by Xiaoyaosan in chronic immobilization-stressed rats. Molecules 2019; 24(3):480. doi: 10.3390/molecules24030480 [Crossref] [ Google Scholar]
- Karaman I. Preprocessing and pretreatment of metabolomics data for statistical analysis. Adv Exp Med Biol 2017; 965:145-61. doi: 10.1007/978-3-319-47656-8_6 [Crossref] [ Google Scholar]
- Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N. HMDB: the human metabolome database. Nucleic Acids Res 2007; 35(Database issue):D521-6. doi: 10.1093/nar/gkl923 [Crossref] [ Google Scholar]
- Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res 2009; 37(Web Server issue):W652-60. doi: 10.1093/nar/gkp356 [Crossref] [ Google Scholar]
- Tabassum I, Siddiqui ZN, Rizvi SJ. Effects of Ocimum sanctum and Camellia sinensis on stress-induced anxiety and depression in male albino Rattus norvegicus. Indian J Pharmacol 2010; 42(5):283-8. doi: 10.4103/0253-7613.70108 [Crossref] [ Google Scholar]
- Rashno M, Ghaderi S, Nesari A, Khorsandi L, Farbood Y, Sarkaki A. Chrysin attenuates traumatic brain injury-induced recognition memory decline, and anxiety/depression-like behaviors in rats: Insights into underlying mechanisms. Psychopharmacology (Berl) 2020; 237(6):1607-19. doi: 10.1007/s00213-020-05482-3 [Crossref] [ Google Scholar]
- Liu J, Lv YW, Shi JL, Ma XJ, Chen Y, Zheng ZQ. Anti-anxiety effect of (-)-syringaresnol-4-O-β-d-apiofuranosyl-(1→2)-β-d-glucopyranoside from Albizzia julibrissin Durazz (Leguminosae). Molecules 2017; 22(8):1331. doi: 10.3390/molecules22081331 [Crossref] [ Google Scholar]
- German-Ponciano LJ, Dutra Costa BP, Feitosa LM, dos Santos Campos K, da Silva Chaves SN, Cueto-Escobedo J. Chrysin, but not flavone backbone, decreases anxiety-like behavior in animal screens. Neurochem Int 2020; 140:104850. doi: 10.1016/j.neuint.2020.104850 [Crossref] [ Google Scholar]
- Wang M, Li T, Xie Y, Zhang D, Qu Y, Zhai S. Clustered health risk behaviors with comorbid symptoms of anxiety and depression in young adults: moderating role of inflammatory cytokines. J Affect Disord 2024; 345:335-41. doi: 10.1016/j.jad.2023.10.139 [Crossref] [ Google Scholar]
- Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H. Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine 2016; 1(1):1003. [ Google Scholar]
- Thorsdottir D, Cruickshank NC, Einwag Z, Hennig GW, Erdos B. BDNF downregulates β-adrenergic receptor-mediated hypotensive mechanisms in the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 2019; 317(6):H1258-71. doi: 10.1152/ajpheart.00478.2019 [Crossref] [ Google Scholar]
- Eckert A, Karen S, Beck J, Brand S, Hemmeter U, Hatzinger M. The link between sleep, stress and BDNF. Eur Psychiatry 2017; 41(S1):S282. doi: 10.1016/j.eurpsy.2017.02.132 [Crossref] [ Google Scholar]
- Angelopoulou E, Pyrgelis ES, Piperi C. Neuroprotective potential of chrysin in Parkinson’s disease: molecular mechanisms and clinical implications. Neurochem Int 2020; 132:104612. doi: 10.1016/j.neuint.2019.104612 [Crossref] [ Google Scholar]
- Afnan A, Saleem A, Akhtar MF. Chrysin, a 5,7-dihydroxyflavone restrains inflammatory arthritis in rats via subsiding oxidative stress biomarkers and inflammatory cytokines. Inflammopharmacology 2023; 31(4):1863-78. doi: 10.1007/s10787-023-01229-6 [Crossref] [ Google Scholar]
- Filho CB, Jesse CR, Donato F, Giacomeli R, Del Fabbro L, da Silva Antunes M. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na + , K + -ATPase activity in the hippocampus and prefrontal cortex of mice: antidepressant effect of chrysin. Neuroscience 2015; 289:367-80. doi: 10.1016/j.neuroscience.2014.12.048 [Crossref] [ Google Scholar]
- Shebl N, El-Jaafary S, Saeed AA, Elkafrawy P, El-Sayed A, Shamma S. Metabolomic profiling reveals altered phenylalanine metabolism in Parkinson’s disease in an Egyptian cohort. Front Mol Biosci 2024; 11:1341950. doi: 10.3389/fmolb.2024.1341950 [Crossref] [ Google Scholar]
- Dong MX, Chen GH, Hu L. Dopaminergic system alteration in anxiety and compulsive disorders: a systematic review of neuroimaging studies. Front Neurosci 2020; 14:608520. doi: 10.3389/fnins.2020.608520 [Crossref] [ Google Scholar]
- Sugama S, Sekiyama K, Kodama T, Takamatsu Y, Takenouchi T, Hashimoto M. Chronic restraint stress triggers dopaminergic and noradrenergic neurodegeneration: possible role of chronic stress in the onset of Parkinson’s disease. Brain Behav Immun 2016; 51:39-46. doi: 10.1016/j.bbi.2015.08.015 [Crossref] [ Google Scholar]
- Buras A, Battle L, Landers E, Nguyen T, Vasudevan N. Thyroid hormones regulate anxiety in the male mouse. Horm Behav 2014; 65(2):88-96. doi: 10.1016/j.yhbeh.2013.11.008 [Crossref] [ Google Scholar]
- Ren Y, Yang CH, Li ZM, Yang Z, Xiao ZJ, Duan JJ. Chronic stress disturbs metabolome of blood plasma and urine in diabetic rats. Front Psychiatry 2018; 9:525. doi: 10.3389/fpsyt.2018.00525 [Crossref] [ Google Scholar]
- Ekeuku SO, Ahmad Shahzalli AB, Tan JK, Makpol S. Untargeted metabolomic profiling on the effect of ginger on rat hepatic changes during ageing. J Funct Foods 2024; 113:106054. doi: 10.1016/j.jff.2024.106054 [Crossref] [ Google Scholar]
- McGowan JC, Hill C, Mastrodonato A, LaGamma CT, Kitayev A, Brachman RA. Prophylactic ketamine alters nucleotide and neurotransmitter metabolism in brain and plasma following stress. Neuropsychopharmacology 2018; 43(9):1813-21. doi: 10.1038/s41386-018-0043-7 [Crossref] [ Google Scholar]
- Hoffman JR, Ostfeld I, Stout JR, Harris RC, Kaplan Z, Cohen H. β-Alanine supplemented diets enhance behavioral resilience to stress exposure in an animal model of PTSD. Amino Acids 2015; 47(6):1247-57. doi: 10.1007/s00726-015-1952-y [Crossref] [ Google Scholar]
- Seino Y, Ohashi N, Kohno T. The endogenous agonist, β-alanine, activates glycine receptors in rat spinal dorsal neurons. Biochem Biophys Res Commun 2018; 500(4):897-901. doi: 10.1016/j.bbrc.2018.04.183 [Crossref] [ Google Scholar]
- Arora I, Mal P, Arora P, Paul A, Kumar M. GABAergic implications in anxiety and related disorders. Biochem Biophys Res Commun 2024; 724:150218. doi: 10.1016/j.bbrc.2024.150218 [Crossref] [ Google Scholar]
- Cochran C, Martin K, Rafferty D, Choi J, Leontyev A, Shetty A. Untargeted metabolomics-based prediction of therapeutic potential for apigenin and chrysin. Int J Mol Sci 2023; 24(4):4066. doi: 10.3390/ijms24044066 [Crossref] [ Google Scholar]
- Liu P, Fleete MS, Jing Y, Collie ND, Curtis MA, Waldvogel HJ. Altered arginine metabolism in Alzheimer’s disease brains. Neurobiol Aging 2014; 35(9):1992-2003. doi: 10.1016/j.neurobiolaging.2014.03.013 [Crossref] [ Google Scholar]
- Maulydia M, Rehatta NM, Soedarmo SM. Effects of glutamine and arginine combination on pro- and anti-inflammatory cytokines. Open Vet J 2023; 13(5):613-9. doi: 10.5455/OVJ.2023.v13.i5.14 [Crossref] [ Google Scholar]
- Keefe P, Puthanveetil P. Compare and contrast of the cellular actions of related flavonoids, apigenin and chrysin. Nutrients 2024; 16(23):4195. doi: 10.3390/nu16234195 [Crossref] [ Google Scholar]