Pharmaceutical Sciences. 2025;31(3):330-337.
doi: 10.34172/PS.025.41026
Research Article
Renal Impact of a High-Loading Dose of Amikacin in Critically Ill Patients
Laleh Mahmoudi Formal analysis, Project administration, 1 
Arezoo Ahmadi Investigation, Project administration, Writing – original draft, 2 
Motahareh Mahi-Birjand Investigation, Writing – original draft, 3 
Effat Alemzadeh Investigation, 4 
Parvin Askari Writing – review & editing, 4 
Ramin Niknam Writing – review & editing, 5 
Mojtaba Mojtahedzadeh Conceptualization, Supervision, 6, * 
Author information:
1Department of Clinical Pharmacy, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
2Department of Anesthesiology & Critical Care, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
3Department of Clinical Pharmacy, School of Pharmacy, Infectious Diseases Research Center, Birjand University of Medical Sciences, Birjand, Iran
4Infectious Diseases Research Center, Birjand University of Medical Sciences, Birjand, Iran
5Gastroenterohepatology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
6Pharmaceutical Research Institute, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Background:
Aminoglycosides are potent bactericidal antibiotics primarily employed for gram-negative infections. However, they face limitations due to potential renal toxicity when administered at high doses. This study aimed to assess the nephrotoxicity of amikacin (AMK) at two distinct dosage levels over seven days in critically ill patients, utilizing renal-specific biomarkers.
Methods:
Critically ill patients with sepsis, severe sepsis, or septic shock were randomly assigned to two treatment groups receiving AMK in combination with a broad-spectrum β-lactam antibiotic according to antibiogram results. Disease severity was assessed using the Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores. Renal function was monitored through serum creatinine (SCr) and kidney injury biomarkers (NGAL and IL-18) measurements.
Results:
Among the 40 patients completing the study, the two groups had no significant differences in APACHE II (P=0.39) and SOFA scores (P=0.30). Baseline plasma creatinine levels exhibited no significant within-group differences. While NGAL levels in group 1(high dose AMK) significantly increased on day 3, no significant differences were observed between the groups in all four measurements (P=0.03). Urinary IL-18 levels within groups demonstrated a significant increase peaking on day 3, with no significant within-group differences over the 7-day follow-up.
Conclusion:
The findings suggest that higher doses of AMK may be administered with a similar effect of low-dose AMK on renal function, as assessed by the RIFLE criteria (Risk, Injury, Failure, Loss, and End-stage kidney disease), a classification system used to evaluate acute kidney injury (AKI) severity in critically ill patients with sepsis.
Keywords: Amikacin, Acute kidney injury, Critically ill patients, Nephrotoxicity, Renal biomarkers, IL-18
Copyright and License Information
© 2025 The Author(s).
This is an open access article and applies the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This work was supported by Tehran University of Medical Sciences and the Institutional Review Board (89-04-33-11814).
Introduction
Aminoglycosides, a class of bactericidal antibiotics, constitute a cornerstone in treating gram-negative infections, especially in severe sepsis or septic shock. Combining aminoglycosides with other antibiotics, particularly broad-spectrum β-lactam antibiotics, is a common therapeutic approach, leveraging synergistic bactericidal effects and broadening the spectrum of activity. Critically ill patients with sepsis often exhibit altered pharmacokinetics, necessitating higher doses of amikacin (AMK) to optimize therapeutic outcomes in patients with severe sepsis or septic shock.1 However, the potential for renal toxicity poses a significant clinical concern with aminoglycoside usage, especially with high aminoglycoside doses. The incidence of nephrotoxicity associated with aminoglycosides ranges from 10% to 25% in critically ill patients, with higher rates reported in those with pre-existing renal impairment or prolonged treatment durations. Notably, nephrotoxicity may occur in up to 30% of patients receiving aminoglycosides for extended periods or at high doses.2
Thus, reasonable administration guided by therapeutic drug monitoring becomes imperative to balance efficacy and minimize adverse effects.3,4 Despite concerns, aminoglycosides, including AMK, remain pivotal in the empiric treatment of gram-negative bacterial infections, particularly against strains resistant to other antibiotics.5,6
The variable nature of renal dysfunction in critically ill septic patients is influenced by multiple factors, 3 which underscores the importance of timely detection of acute kidney injury (AKI). Specific renal biomarkers offer valuable insights for early intervention.7,8 The RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) classification provides a comprehensive framework for scoring and categorizing AKI severity, relying on assessment of serum creatinine (SCr) levels, glomerular filtration rates (GFR), or urine output.9 Recognizing limitations in SCr’s predictive value, novel kidney-specific biomarkers such as interleukin-18 (IL-18) and neutrophil gelatinase-associated lipocalin (NGAL) have emerged for early AKI detection.10,11 These biomarkers can identify kidney damage events 24-48 hours earlier than SCr with acceptable sensitivity. NGAL, a 25 kDa protein, exhibits a rapid release within 2 hours of kidney epithelial cell damage, aiding in the early identification of ischemic and nephrotoxic AKI.11-15 IL-18, a pro-inflammatory cytokine, elevates during instances of innate inflammation and in patients experiencing AKI. Research has revealed that the levels of this dependable biomarker tend to rise 24-48 hours before the noticeable increase in SCr levels, offering a valuable lead time for intervention. AMK primarily induces nephrotoxicity by accumulating in the proximal tubular epithelial cells, leading to oxidative stress, mitochondrial dysfunction, and tubular cell apoptosis. NGAL is an early and sensitive biomarker of tubular damage, as it is rapidly upregulated in response to kidney injury, particularly in cases of ischemic and nephrotoxic AKI. Similarly, IL-18 is a pro-inflammatory cytokine released from proximal tubules in response to injury, providing a reliable marker of inflammatory damage associated with aminoglycoside nephrotoxicity. These biomarkers allow for earlier detection of kidney injury compared to traditional markers like SCr, thereby enhancing the ability to monitor nephrotoxicity in critically ill patients.8,12-18
Despite the importance of AMK, limited research has explored doses exceeding 25 mg/kg.1,19 Notably, the renal effects of high-dose AMK, particularly those surpassing 25 mg/kg, remain unexplored.
This study addresses this gap, aiming to evaluate nephrotoxicity associated with high-dose AMK regimen compared to the conventional doses in critically ill patients with sepsis. In addition to SCr, this investigation incorporates NGAL and IL-18 as direct markers of tubular injury, providing a comprehensive understanding of renal function in this clinical context.
Methods
Study design
This double-blind randomized trial was conducted within a general intensive care unit (ICU), serving as a tertiary referral center for trauma patients in Tehran, the capital city of Iran, between April 2016 and May 2017.
Sampling and patient blinding
The sample size, calculated with a significance level (α) of 0.05 and power (1-β) of 80%, determined that 20 patients per group were required. Patients meeting eligibility criteria were allocated to intervention groups using blocked randomization in a 1:1 ratio. To ensure unbiased results, both patients and investigators remained blinded to specific antibiotic regimens throughout the experiment.
Exclusion and inclusion criteria
To be eligible for participation in the study, patients were required to have sepsis necessitating combination antibiotic therapy, including AMK, and expected to require antibacterial therapy for longer than 7 days. Male and female patients aged 18 to 65, with a body mass index (BMI) < 30 kg/m2, and normal SrCr level ( ≤ 1.2 mg/dL), and eGFR ≥ 60 mL/min/1.73 m2 were included. A BMI threshold of < 30 kg/m2 was chosen to minimize the impact of altered aminoglycoside pharmacokinetics in obese patients, as increased adipose tissue can affect drug distribution and clearance. Criteria for severe sepsis and septic shock followed Surviving Sepsis Campaign guidelines.20 Exclusion criteria encompassed conditions such as rapidly progressive AKI within the initial 72 hours, major baseline comorbidities, extreme body weights (BMI > 35 or < 18.5), age below 18 or over 65, and anticipated survival less than the study period, major cardiac, pulmonary, hepatic disorders, neuromuscular diseases, and pregnancy.
Intervention
The patients were divided randomly into two treatment groups with different doses of AMK 25 mg/kg/d (Group 1) and a conventional dose of 15 mg/kg/d (Group 2). The initial dose of AMK was determined based on the total body weight of patients with a BMI below 30 kg/m2. Finally, 2 groups of selected patients were compared for the occurrence of nephrotoxicity.
Patients were assigned to treatment groups receiving AMK in combination with a broad-spectrum β-lactam antibiotic. Two groups received different doses: Group 1 (25 mg/kg/d) and group 2 (standard 15 mg/kg/d), based on adjusted body weight. Dosages were categorized as high or low based on desired maximum concentration (Cmax) goals. All individuals received standard treatments and care by the decisions made by the medical staff.
Measurements and study outcomes
Upon admission, patient characteristics and medical history were documented, with disease severity assessed using Acute Physiology and Chronic Health Evaluation (APACHE) II21 and Sequential Organ Failure Assessment (SOFA) scores.22 The study compared nephrotoxicity profiles over a 7-day course, focusing on AKI and SOFA scores. Renal function was evaluated using RIFLE criteria.9 During the ICU stay, daily SOFA scores, AKI assessments, and documentation of renal replacement therapy and nephrotoxic drug administration (such as glycopeptides and diuretics) were conducted.
Renal function assay
Serum and urine levels of IL-18 and NGAL were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits. The lower limit of detection for IL-18 was 3.5 pg/mL, with intra-assay and inter-assay coefficients of variation (CVs) of approximately 5.0% and 7.5%, respectively. For NGAL, the assay sensitivity was 0.012 pg/mL, with intra-assay and inter-assay CVs of 4.0% and 6.5%. All samples were aliquoted and stored at -80 °C prior to analysis to prevent degradation. Repeated freeze-thaw cycles were avoided to maintain assay integrity.23 Urine and blood samples (5 mL) were taken before AMK (baseline) as well as on days 1, 3, 5 and 7 after AMK administration. Following blood centrifugation, serum and urine samples were stored at -80 °C. SCr, NGAL, and IL-18 were measured on days 1, 3, 5 and 7 after starting AMK.
NGAL assay
Diluted samples were added to each well. Microtitre plates were then incubated for 1 hour at room temperature and then washed out with buffer. The plates were incubated with biotinylated anti-human NGAL monoclonal antibody, followed by treatment with avidin-conjugated horseradish peroxidase for 1 hour. Tetra methyl benzidine substrate was added to each well in the dark chamber for 10 minutes, then read using an ELISA reader at a wavelength of 450 nm with a reference filter of 620 nm.
IL-18
The measurement of human IL-18 levels in urine was conducted using a commercially available assay kit, specifically the Human IL-18 ELISA Kit from Bender Med Systems GmbH in Vienna, Austria, according to the manufacturer’s instructions.
Adverse effects
Drug safety assessments included side effects other than renal complications, including laboratory findings, and physical examinations. These side effects were defined as drug-related outcomes that started during or after the first dose of the study drug.
Adverse event monitoring
Patients were monitored daily for potential adverse events throughout the 7-day study period. Laboratory parameters including SCr, NGAL, and IL-18 were measured at baseline and on days 3, 5, and 7 to detect signs of nephrotoxicity or AKI. Adverse renal events were classified based on the RIFLE criteria. Non-renal adverse events (e.g., ototoxicity, vestibular symptoms) were assessed clinically and documented if reported.
Statistical analysis
Statistical analyses were conducted using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics were calculated for all variables included in the study. To ensure the normality of the distribution of continuous variables, a Kolmogorov-Smirnov test was employed, and histograms and normal quantile plots were examined. Discrete variables were presented as counts (percentage), while continuous variables were expressed as mean ± standard deviation (SD). The demographic and clinical differences between the study groups were assessed using appropriate statistical tests such as the χ2 test, Fisher’s exact test, student’s t-test, or the Mann-Whitney U test, based on the nature of the variables being compared. This pilot study achieved 80% power (β = 0.2) at alpha = 0.05 to detect differences.
Results
Patients’ characteristics
Out of 307 patients admitted to the ICU of Sina Hospital between April 2016 and May 2017, 80 (26.06%) received AMK for severe sepsis or septic shock based on antibiotic sensitivity results. Among them, 40 met the inclusion criteria and completed the study (Figure 1).
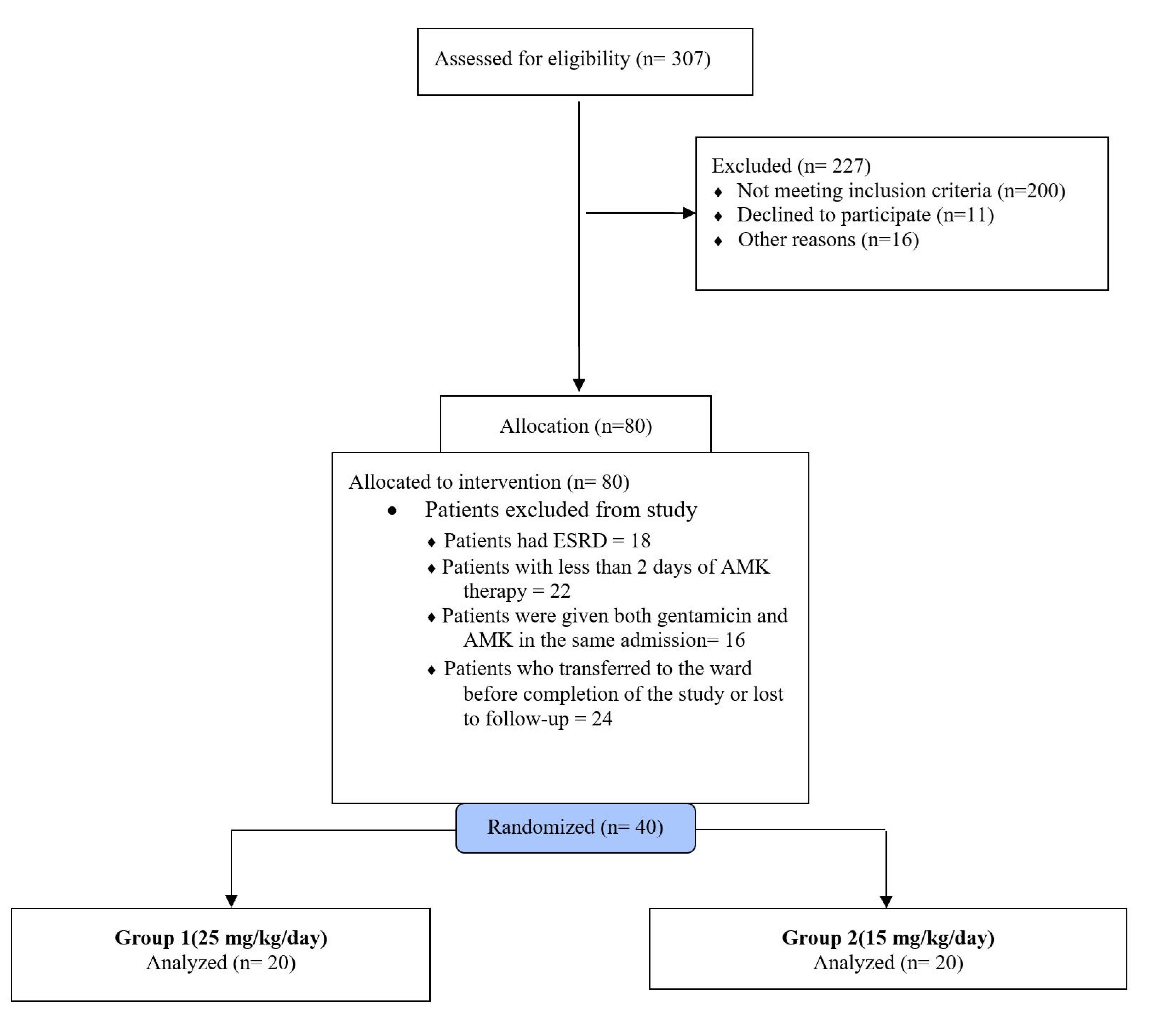
Figure 1.
The Study flow diagram of all patients screened and excluded patients
.
The Study flow diagram of all patients screened and excluded patients
Table 1 summarizes the patient characteristics on ICU admission. No significant differences were observed in APACHE II score (Group 1: 18.45 ± 5.54, Group 2: 15 ± 5; P = 0.39) or SOFA scores on days 1 (Group 1: 10 ± 3, Group 2: 11 ± 3; P = 0.30) between the two groups. The mean maximum (Cmax) and minimum (Cmin) serum concentrations of AMK were measured. In group 1, Cmax was 32.30 ± 13.11 μg/mL, and Cmin was 4.15 ± 2.09 μg/mL. In group 2, the mean Cmax was 49.17 ± 16.35 μg/mL, and the mean Cmin was 4.95 ± 2.56 μg/mL.
Table 1.
Included patients’ characteristics
|
Parameter
|
Group 1
(25 mg/kg/d)
|
Group 2
(15 mg/kg/d)
|
P
value
|
| Patient demographics |
|
|
|
| Number of patients |
20 |
20 |
|
| Age (years) |
43.55 + 8.51 |
49.11 ± 9.49 |
0.072 |
| Sex (male)/(female) |
12/8 |
14/6 |
0.69 |
| Weight (kg) |
69.65 + 8.97 |
65.03 ± 10.21 |
0.14 |
| No. of patients on mechanical ventilation |
18 |
16 |
0.66 |
| Urine output (mL/24 h) |
3887.50 + 1131.47 |
3540 + 1520 |
0.42 |
| Isolated pathogen (%) |
|
|
|
| Pseudomonas aeruginosa |
58 |
53 |
0.44 |
| Acinetobacter baumannii |
13 |
11 |
0.36 |
| Escherichia coli |
15 |
17 |
0.40 |
| Others gram-negative bacteria |
14 |
19 |
0.33 |
| Monitoring data |
|
|
|
| APACHE II score |
18.45 ±5.54 |
15 ± 5 |
0.39 |
| SOFA score day 1 |
10 ± 3 |
11 ± 3 |
0.30 |
| SOFA score day 3 |
9 ±3 |
10 ±3 |
0.30 |
| SOFA score day 5 |
8 ±3 |
7 ±3 |
0.50 |
| SOFA score day 7 |
6 ±2 |
7 ±1520 |
0.12 |
| Inotrope/vasopressor support (%) |
12 (60%) |
10 (50%) |
0.44 |
| Sites of infection (%) |
|
|
0.98 |
| Pulmonary |
9 (45%) |
8 (40%) |
|
| Catheter |
4 (20%) |
5 (25%) |
|
| Urinary tract |
4 (20%) |
4 (20%) |
|
| Bacteraemia |
3 (15%) |
3(15%) |
|
APACHE, Acute Physiology, and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment.
Renal function assessment
Table 2 presents SCr levels on days 1, 3, 5, and 7. Baseline SCr levels on day 1 were comparable between group 1 (1.15 ± 0.89 mg/dL) and group 2 (1.10 ± 1.10 mg/dL) with no significant difference (P = 0.95). Moreover, SCr levels on days 3, 5, and 7 showed no significant differences between the two groups throughout the 7-day follow-up (P = 0.87). Within-group changes in SCr levels over timewere also evaluated, but no significant trends were observed in either group.
Table 2.
Serum creatinine (SCr) in two study groups, follow-up for 7 days
|
Creatinine (mg/dL)
|
Group 1
(25 mg/kg/d)
|
Group 2
(15 mg/kg/d)
|
P
value*
|
| Day 1(admission) |
1.15 ± 0.89 |
1.10 ± 1.10 |
0.95 |
| Day 3 |
1.6 + 0.75 |
1.6 ± 0.65 |
0.15 |
| Day 5 |
1.7 ± 0.97 |
1.55 ± 0.72 |
1 |
| Day 7 (admission) |
1.87 ± 1.14 |
1.89 ± 1.19 |
0.87 |
|
P value** |
0.58 |
0.83 |
|
Test: Repeated measures ANOVA.
* Comparison between groups 1 and 2; ** Within each group.
NGAL levels in group 1 showed a significant increase on day 3 compared to baseline (P = 0.03). Still, no significant difference was observed between the groups across all time points (P = 0.76, Table 3). In group 2, NGAL levels did not show significant variation during the study period (P = 0.53).
Table 3.
Neutrophil gelatinase-associated lipocalin (NGAL) in two study groups, follow-up for 7 days
|
NGAL (ng/mL)
|
Group 1
(25 mg/kg/d)
|
Group 2
(15 mg/kg/d)
|
P
value*
|
| Day 1 (admission) |
156.16 ± 120.60 |
148.15 ± 58.19 |
0.4 |
| Day 3 |
180.96 ± 135.69 |
161.91 ± 122.72 |
0.76 |
| Day 5 |
160.90 ± 120.60 |
156.52 ± 75.80 |
0.07 |
| Day 7 |
168.05 ± 106.48 |
153.94 ± 81.70 |
0.3 |
|
P value** |
0.03 |
0.53 |
|
Test: Repeated measures ANOVA.
* Comparison between groups 1 and 2; ** Within each group.
The urinary levels of IL-18 significantly increased within each group on day 3 (P = 0.045 in group 1, P = 0.028 in group 2), peaking on day 3. However, there was no significant difference in IL-18 levels between the two groups during the 7-day follow-up (P = 0.38, Table 4).
Table 4.
Interleukin 18 (IL-18) in two study groups, follow-up for 7 days
|
IL-18 (pg/mL) |
Group 1
(25 mg/kg/d)
|
Group 2
(15 mg/kg/d)
|
P
value*
|
| Day 1 (admission) |
449.53 ± 253.41 |
304.93 ± 166.22 |
0.04 |
| Day 3 |
687.74 ± 335.43 |
682.46 + 511.46 |
0.97 |
| Day 5 |
656.21 ± 424.26 |
674.23 ± 455.20 |
0.90 |
| Day 7 |
620.74 ± 463.81 |
448.99 ± 282.52 |
0.38 |
|
P value** |
0.045 |
0.028 |
|
Test: Repeated measures ANOVA.
* Comparison between groups 1 and 2; ** Within each group.
In this study, AKI was evaluated according to the RIFLE criteria. AKI was defined as an increase in SCr by ≥ 0.3 mg/dL or a 50% increase from baseline. A total of 5 patients (25%) in group 1 (25 mg/kg/d) met the RIFLE criteria for AKI during the follow-up period. The majority of these patients experienced a mild increase in SCr. No patients in this group progressed to the higher categories of AKI. In group 2 (15 mg/kg/d), 4 patients (20%) developed AKI, with most cases falling under the risk category. Similar to group 1, no patients in group 2 progressed to the injury or failure categories.
Adverse Events
Among the patients in group 1, one exhibited eosinophilia, and in each group, one patient developed a skin rash. These adverse events did not require discontinuation of AMK treatment for any participant. Due to the limited number of patients with AKI (n = 9), statistical comparisons between AKI and non-AKI groups for biomarker levels were presented descriptively without formal subgroup analysis (Table 5).
Table 5.
Comparison of NGAL and IL-18 in patients with and without AKI on Day 3
|
Biomarker
|
AKI (n=9)
|
Non-AKI (n=31)
|
P
value*
|
| NGAL (ng/mL) |
195.0 ± 140.0 |
160.0 ± 120.0 |
0.25* |
| IL-18 (pg/mL) |
710.0 ± 330.0 |
660.0 ± 400.0 |
0.40* |
Test: Repeated measures ANOVA
* Comparison between groups 1 and 2.
Discussion
Despite the potential benefits of higher doses of aminoglycosides in achieving therapeutic targets, a key concern associated with aminoglycosides, including AMK, is the increased risk of nephrotoxicity. The incidence of nephrotoxicity with aminoglycosides in critically ill patients ranges from 10% to 25%, with higher rates observed in those with pre-existing renal impairment or prolonged treatment durations. This variation in nephrotoxicity rates highlights the challenge in assessing the true risk of kidney injury associated with aminoglycosides. In our present study, the primary aim was to investigate the correlation between the severity of renal injury and the administration of two distinct loading doses of AMK: the conventional 15 mg/kg and a higher dose of 25 mg/kg/d. We evaluated this relationship by measuring renal biomarkers IL-18 and NGAL, both known for their high specificity and sensitivity in the early detection of renal damage in critically ill patients. This finding suggests the potential to utilize the higher dose of AMK to achieve a higher Cmax while maintaining a comparable level of nephrotoxicity, as seen with the conventional dose. Critically ill patients exhibit considerable heterogeneity due to variations in underlying diseases, disease severity, and organ dysfunction.1 Dosing regimens in ICUs are often extrapolated from clinical trials involving non-ICU patients or healthy individuals, assuming comparable pharmacokinetic variables between these groups. However, septic patients may require higher doses of aminoglycosides due to physiological changes resulting from sepsis, stress, and the impact of hemodynamic and medical interventions.1 The potential nephrotoxicity of AMK, particularly at higher doses, remains a concern for sepsis treatment in ICU patients.24
To assess nephrotoxicity in this study, we chose IL-18 and NGAL as biomarkers due to their ability to detect renal injury earlier than traditional markers like SCr. AMK-induced nephrotoxicity primarily occurs via accumulation in proximal tubular epithelial cells, leading to oxidative stress, mitochondrial dysfunction, and tubular cell apoptosis. NGAL is rapidly upregulated in response to kidney injury, especially in cases of ischemic and nephrotoxic AKI. Similarly, IL-18 is a pro-inflammatory cytokine released from proximal tubules following injury, serving as a reliable marker for the inflammatory damage associated with aminoglycoside nephrotoxicity.9-11 Our findings show that administration of 25 mg/kg of AMK, compared to 15 mg/kg, did not result in an increased incidence of acute renal injury when assessed based on SCr levels according to the RIFLE criteria. However, urinary NGAL levels showed a significant increase on day 3 (P = 0.03) in group 1, with no significant differences observed between the two groups in all other measurements. These results, in conjunction with the lack of significant difference in SCr levels, suggest that both doses of AMK exert a similar renal impact. Notably, in cases without a significant increase in SCr, an elevation in NGAL on day 3 may serve as an early predictor of AMK’s renal effects, similar to findings in premature neonates during gentamicin treatment, where significant elevations in NGAL were observed even without a significant rise in SCr.24,25
In an animal study, plasma and urine concentrations of NGAL increased 4- to 38-fold following AMK infusion.26 Further studies, such as those investigating serum NGAL in high-dose methotrexate-induced AKI, have concluded that NGAL is an effective marker for identifying direct tubular damage caused by nephrotoxic agents.27 Regarding IL-18, urinary levels showed a significant increase (P = 0.045 and 0.028 in groups 1 and 2, respectively) on day 3. Despite this, no significant difference was observed between the two groups over the 7-day follow-up (P = 0.38). These results indicate that both doses of AMK had a similar renal impact. However, the elevation in IL-18 levels on day 3 may reflect the presence of AKI that could manifest in the subsequent days, as SCr levels remained within the normal range. The increase in IL-18 levels suggests enhanced toxicity associated with both doses of AMK, consistent with findings from other studies that have linked IL-18 to early kidney injury, such as in pediatric chemotherapy patients and after cardiopulmonary bypass surgery.28-30 Although these findings are valuable, we must interpret them with caution, as no difference in toxicity was observed based on SCr and NGAL biomarkers. It should be noted that there are very few studies on the renal effects of high-dose AMK in ICU patients, which limits the ability to compare our findings directly with existing literature. However, a study by Bartal et al. demonstrated that standard doses of AMK did not result in significant differences in renal biomarker levels between patients with renal dysfunction and those with normal renal function.31
In previous studies, patients with severe sepsis have been recommended doses of AMK exceeding 25 mg/kg for individuals with a suspected increased volume of distribution (Vd), such as ICU patients.1,26 Peak AMK levels below 40 μg/mL are associated with worse outcomes,32 aligning with reports that suggest higher doses (e.g., 25-30 mg/kg) are necessary to achieve target levels effective against more resistant bacteria in ICU settings.33,34 In our study, almost all patients receiving 15 mg/kg/d of AMK had a peak level (Cmax) below 40 μg/mL, while the 25 mg/kg/d dose helped achieve this threshold. This is unsurprising, given that Vd in critically ill patients is significantly increased compared to the general population, influencing serum AMK levels.1 Notably, no impairment in renal function was observed with the higher dose, likely because the minimum concentration (Cmin) in both groups was below the threshold of toxicity (5 μg/mL).35
In summary, our study suggests that the higher dose of AMK (25 mg/kg per day) could be considered a viable option for achieving the desired therapeutic levels in critically ill patients, with renal injury outcomes comparable to those observed with the conventional dose. This insight may inform AMK dosing strategies in ICU settings, where combating more resistant bacteria is a critical concern. Furthermore, our data indicate that AKI biomarkers, particularly IL-18 and NGAL, may increase during AMK treatment even before changes in SCr are observed, suggesting their potential as early predictors of renal failure. However, further studies are needed to confirm this hypothesis. Several limitations should be acknowledged. First, the relatively small sample size may limit the generalizability of our findings to a broader patient population. Second, the seven-day follow-up period may not capture long-term effects of AMK treatment, especially given that treatments beyond 10 days are known to carry a higher risk of nephrotoxicity. Third, this study was not designed to assess the pharmacokinetic parameters associated with AMK dosing. Lastly, as previously mentioned, the variability in nephrotoxicity incidence across different studies highlights the need for a larger cohort to detect the true incidence and pattern of nephrotoxicity biomarkers in our population.
Conclusion
In conclusion, our study suggests that high-dose AMK (25 mg/kg/d) may not result in significantly higher nephrotoxicity compared to the conventional dose in critically ill patients. However, careful monitoring of kidney function, including biomarkers like IL-18 and NGAL, is essential when using high doses of AMK. Further research with larger sample sizes is necessary to fully understand the safety and efficacy of higher doses in this patient population.
Competing Interests
The authors declare no competing interests.
Consent to Participate
Outlined in the Declaration of Helsinki. Informed consent was obtained from either the patients or their first-degree relatives, and each patient was included only once.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Ethical Approval
Approval for the study was obtained from the Ethics Committee of Tehran University of Medical Sciences and the Institutional Review Board (89-04-33-11814). The study was registered under the clinical trial registration number IRCT2012091110817N1 and adhered to the principles.
Acknowledgements
In general, the ICU of Sina Hospital is affiliated to the Tehran University of medical sciences. The authors would like to thank all participants, as well as the staff who supported it.
References
- Mahmoudi L, Mohammadpour AH, Ahmadi A, Niknam R, Mojtahedzadeh M. Influence of sepsis on higher daily dose of amikacin pharmacokinetics in critically ill patients. Eur Rev Med Pharmacol Sci 2013; 17(3):285-91. [ Google Scholar]
- Chou CL, Chuang NC, Chiu HW, Liao CT, Hsu YH, Chang TH. Aminoglycosides use has a risk of acute kidney injury in patients without prior chronic kidney disease. Sci Rep 2022; 12(1):17212. doi: 10.1038/s41598-022-21074-x [Crossref] [ Google Scholar]
- Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 1987; 155(1):93-9. doi: 10.1093/infdis/155.1.93 [Crossref] [ Google Scholar]
- Begg EJ, Barclay ML, Kirkpatrick CM. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol 2001; 52(Suppl 1):35S-43S. doi: 10.1046/j.1365-2125.2001.0520s1035.x [Crossref] [ Google Scholar]
- Eyler RF, Mueller BA. Antibiotic dosing in critically ill patients with acute kidney injury. Nat Rev Nephrol 2011; 7(4):226-35. doi: 10.1038/nrneph.2011.12 [Crossref] [ Google Scholar]
- Duszynska W, Taccone FS, Hurkacz M, Kowalska-Krochmal B, Wiela-Hojeńska A, Kübler A. Therapeutic drug monitoring of amikacin in septic patients. Crit Care 2013; 17(4):R165. doi: 10.1186/cc12844 [Crossref] [ Google Scholar]
- Van Biesen W, Vanholder R, Lameire N. Defining acute renal failure: RIFLE and beyond. Clin J Am Soc Nephrol 2006; 1(6):1314-9. doi: 10.2215/cjn.02070606 [Crossref] [ Google Scholar]
- Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol 2005; 16(10):3046-52. doi: 10.1681/asn.2005030236 [Crossref] [ Google Scholar]
- Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J 2013; 6(1):8-14. doi: 10.1093/ckj/sfs160 [Crossref] [ Google Scholar]
- Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 2004; 43(3):405-14. doi: 10.1053/j.ajkd.2003.10.040 [Crossref] [ Google Scholar]
- Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003; 14(10):2534-43. doi: 10.1097/01.asn.0000088027.54400.c6 [Crossref] [ Google Scholar]
- Adiyanti SS, Loho T. Acute kidney injury (AKI) biomarker. Acta Med Indones 2012; 44(3):246-55. [ Google Scholar]
- Makris K, Markou N, Evodia E, Dimopoulou E, Drakopoulos I, Ntetsika K. Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med 2009; 47(1):79-82. doi: 10.1515/cclm.2009.004 [Crossref] [ Google Scholar]
- Devarajan P. Neutrophil gelatinase-associated lipocalin—an emerging troponin for kidney injury. Nephrol Dial Transplant 2008; 23(12):3737-43. doi: 10.1093/ndt/gfn531 [Crossref] [ Google Scholar]
- Washburn KK, Zappitelli M, Arikan AA, Loftis L, Yalavarthy R, Parikh CR. Urinary interleukin-18 is an acute kidney injury biomarker in critically ill children. Nephrol Dial Transplant 2008; 23(2):566-72. doi: 10.1093/ndt/gfm638 [Crossref] [ Google Scholar]
- Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009; 54(6):1012-24. doi: 10.1053/j.ajkd.2009.07.020 [Crossref] [ Google Scholar]
- Liu Y, Guo W, Zhang J, Xu C, Yu S, Mao Z. Urinary interleukin 18 for detection of acute kidney injury: a meta-analysis. Am J Kidney Dis 2013; 62(6):1058-67. doi: 10.1053/j.ajkd.2013.05.014 [Crossref] [ Google Scholar]
- Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol 2008; 48:463-93. doi: 10.1146/annurev.pharmtox.48.113006.094615 [Crossref] [ Google Scholar]
- Roger C, Nucci B, Louart B, Friggeri A, Knani H, Evrard A. Impact of 30 mg/kg amikacin and 8 mg/kg gentamicin on serum concentrations in critically ill patients with severe sepsis. J Antimicrob Chemother 2016; 71(1):208-12. doi: 10.1093/jac/dkv291 [Crossref] [ Google Scholar]
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41(2):580-637. doi: 10.1097/ccm.0b013e31827e83af [Crossref] [ Google Scholar]
- Gunning K, Rowan K. ABC of intensive care: outcome data and scoring systems. BMJ 1999; 319(7204):241-4. doi: 10.1136/bmj.319.7204.241 [Crossref] [ Google Scholar]
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22(7):707-10. doi: 10.1007/bf01709751 [Crossref] [ Google Scholar]
- Abu El-Makarem MA, Mahmoud YZ, Moussa MM, El-Saghir SM, Keryakos HK. Do old urinary biomarkers have a place in the new definition of hepatorenal syndrome in the Egyptian cirrhotic patients? A single-center experience. Egypt Liver J 2022; 12(1):23. doi: 10.1186/s43066-022-00185-0 [Crossref] [ Google Scholar]
- Najmeddin F, Ahmadi A, Mahmoudi L, Sadeghi K, Khalili H, Ahmadvand A. Administration of higher doses of amikacin in early stages of sepsis in critically ill patients. Acta Med Iran 2014; 52(9):703-9. [ Google Scholar]
- McWilliam SJ, Antoine DJ, Sabbisetti V, Turner MA, Farragher T, Bonventre JV. Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: a proof-of-concept study. PLoS One 2012; 7(8):e43809. doi: 10.1371/journal.pone.0043809 [Crossref] [ Google Scholar]
- Studená Š, Doleželová E, Cermanová J, Prašnická A, Springer D, Mičuda S. Evaluation of neutrophil gelatinase-associated lipocalin as a predictor of glomerular filtration rate and amikacin clearance during early rat endotoxemia: comparison with traditional endogenous and exogenous biomarkers. Eur J Drug Metab Pharmacokinet 2020; 45(1):71-80. doi: 10.1007/s13318-019-00579-3 [Crossref] [ Google Scholar]
- Li H, Xu Q, Wang Y, Chen K, Li J. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for predicting high dose methotrexate associated acute kidney injury in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 2020; 85(1):95-103. doi: 10.1007/s00280-019-03980-6 [Crossref] [ Google Scholar]
- Zubowska M, Wyka K, Fendler W, Młynarski W, Zalewska-Szewczyk B. Interleukin 18 as a marker of chronic nephropathy in children after anticancer treatment. Dis Markers 2013; 35(6):811-8. doi: 10.1155/2013/369784 [Crossref] [ Google Scholar]
- Liang XL, Liu SX, Chen YH, Yan LJ, Li H, Xuan HJ. Combination of urinary kidney injury molecule-1 and interleukin-18 as early biomarker for the diagnosis and progressive assessment of acute kidney injury following cardiopulmonary bypass surgery: a prospective nested case-control study. Biomarkers 2010; 15(4):332-9. doi: 10.3109/13547501003706558 [Crossref] [ Google Scholar]
- Koyner JL, Coca SG, Thiessen-Philbrook H, Patel UD, Shlipak MG, Garg AX. Urine biomarkers and perioperative acute kidney injury: the impact of preoperative estimated GFR. Am J Kidney Dis 2015; 66(6):1006-14. doi: 10.1053/j.ajkd.2015.07.027 [Crossref] [ Google Scholar]
- Bartal C, Danon A, Schlaeffer F, Reisenberg K, Alkan M, Smoliakov R. Pharmacokinetic dosing of aminoglycosides: a controlled trial. Am J Med 2003; 114(3):194-8. doi: 10.1016/s0002-9343(02)01476-6 [Crossref] [ Google Scholar]
- Moore RD, Smith CR, Lietman PS. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med 1984; 77(4):657-62. doi: 10.1016/0002-9343(84)90358-9 [Crossref] [ Google Scholar]
- Gálvez R, Luengo C, Cornejo R, Kosche J, Romero C, Tobar E. Higher than recommended amikacin loading doses achieve pharmacokinetic targets without associated toxicity. Int J Antimicrob Agents 2011; 38(2):146-51. doi: 10.1016/j.ijantimicag.2011.03.022 [Crossref] [ Google Scholar]
- Taccone FS, Laterre PF, Spapen H, Dugernier T, Delattre I, Layeux B. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care 2010; 14(2):R53. doi: 10.1186/cc8945 [Crossref] [ Google Scholar]
- Tod M, Lortholary O, Seytre D, Semaoun R, Uzzan B, Guillevin L. Population pharmacokinetic study of amikacin administered once or twice daily to febrile, severely neutropenic adults. Antimicrob Agents Chemother 1998; 42(4):849-56. doi: 10.1128/aac.42.4.849 [Crossref] [ Google Scholar]