Pharmaceutical Sciences. 2025;31(3):322-329.
doi: 10.34172/PS.025.41013
Research Article
The Effects of Probiotic Supplementation on Level of Disability, Depressive Symptoms, and Cognitive Outcomes in Relapsing-Remitting Multiple Sclerosis Patients: A Randomized Double-Blind Placebo-Controlled Trial
Amirreza Naseri Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, 1 
Sarvin Sanaie Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing, 2 
Sama Rahnemayan Data curation, Investigation, Visualization, Writing – original draft, 2 
Reza Mosaddeghi-Heris Data curation, Investigation, Visualization, Writing – original draft, 2 
Malihe Talebi Investigation, Writing – review & editing, 2
Mahnaz Talebi Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing, 2, * 
Author information:
1Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
2Neurosciences Research Center (NSRC), Imam Reza General Hospital, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Multiple sclerosis (MS) is a chronic autoimmune disease characterized by inflammation and demyelination of the central nervous system. Probiotics, through the gut-brain axis, are suggested to enhance clinical outcomes in patients with MS. This study scrutinizes the effects of probiotic supplementation in relapsing-remitting MS (RRMS) patients.
Methods:
In this parallel, randomized, double-blind, placebo-controlled trial, 90 RRMS patients, with Expanded Disability Status Scale (EDSS)<4, received either the probiotic (Lactocare®) or a placebo twice daily for four months. Assessed outcomes included level of disability (based on EDSS), cognitive function (Symbol Digit Modalities Test [SDMT], three-second version of Paced Auditory Serial Addition Test [PASAT-3]), depressive symptoms (Beck Depression Inventory-II [BDI-II]), and manual dexterity (Nine-Hole Peg Test [9HPT]). Blinding was performed for outcome assessors and the patients. All assessments were conducted at baseline and after four months, and the findings compared between the groups of the study.
Results:
Out of 90 randomized patients, 60 completed the trial (29 in the probiotics group, 31 in the placebo group). Probiotics supplementation was not associated with significant improvement in EDSS, BDI-II, PASAT, SDMT, and non-dominant hand 9HPT (p-values>0.05). Intragroup improvements in PASAT-3 (change median: 2 [IQR:9.5]) and dominant hand 9HPT (change median: -0.43 [IQR: 2.15]) were observed in the probiotic supplementation group, which was comparable to placebo.
Conclusion:
Supplementation with a seven-strain probiotics product for four months does not result in a significant improvement in depressive symptoms, cognitive performance, level of disability, and manual dexterity of RRMS patients with EDSS<4.
Keywords: Multiple sclerosis, Probiotics, Cognitive function, Depressive symptoms, Physical disability, Randomized controlled trial
Copyright and License Information
© 2025 The Author(s).
This is an open access article and applies the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This study was financially supported by Tabriz University of Medical Sciences (registration code: 69811).
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease characterized by inflammation and demyelination of the central nervous system, leading to a broad range of physical, cognitive, and emotional impairments.1,2 The most common phenotype of MS is relapsing-remitting (RRMS).3 Depressive symptoms and neuropsychiatric dysfunction affect up to 70% of MS patients and can significantly impact quality of life and treatment adherence.4-6 Additionally, physical disabilities, such as gait disturbances and hand dexterity issues, are major contributors to the disease burden.7,8 As MS progresses, managing these diverse symptoms becomes increasingly challenging,9 which highlights the importance of prompt diagnosis and management of MS.
Recent attention has focused on the gut-brain axis, highlighting the connection between gut microbiota and neurological health.10,11 Probiotics, defined as live microorganisms that confer health benefits when consumed in adequate amounts, are believed to influence this axis, potentially improving cognitive function, emotional well-being, and physical abilities.12-15 In MS patients, dysbiosis, or microbial imbalance, has been linked to worsened disease outcomes, suggesting that probiotic supplementation could offer therapeutic benefits.16 Probiotics may influence cognitive and physical health, as well as psychological well-being, in MS patients.17 Research has shown that probiotics may enhance motor function in MS animal models, reduce depressive symptoms, and mitigate cognitive decline in various populations.18-20
Despite growing evidence of the potential benefits of probiotics supplementation in neurological and psychological health, its clinical effects on MS patients remain minimally explored. A meta-analysis of the randomized controlled trials (RCTs), reported the beneficial effects of probiotics in improving the mental health of MS patients; however, very low certainty of evidence, suggested more studies on this topic.21 This RCT aimed to evaluate the impact of probiotic supplementation on cognitive function, depressive symptoms, level of disability, and hand dexterity in RRMS patients with Expanded Disability Status Scale (EDSS) < 4.
Patients and Methods
Study design
This study was a parallel, randomized, double-blind, placebo-controlled clinical trial designed to evaluate the effects of probiotics supplementation on the clinical outcomes of patients with RRMS, and the final article was reported following the Consolidated Standards of Reporting Trials (CONSORT) statement.22 The allocation ratio for this trial was 1:1.
Participants
Inclusion criteria were confirmed diagnosis of RRMS according to the 2017 MacDonald criteria,3 the ability to unaided walk (EDSS < 4) along with the willingness to provide informed consent. Exclusion criteria included the presence of other neurological disorders in addition to MS, diabetes, anemia, other inflammatory or rheumatologic conditions, acute or chronic infections, relapse of the disease in the last three months, recent use of corticosteroid pulse therapy, thyroid diseases, discopathies, diagnosed depression under antidepressants therapy, substance abuse, pregnancy, low educational level (below high school, twelve-years) or insufficient proficiency in the Persian language. Out of 122 screened patients for participation in this trial, finally, 45 eligible patients were randomized into each group of the study. Patients were retrieved from a MS clinic, affiliated with the Tabriz University of Medical Sciences (TUOMS).
Intervention
The intervention group received the probiotic supplement (Lactocare®, contains 2 × 109 CFU of Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, Streptococcus thermophiles species, andprebiotic fructo-oligosaccharide) produced by Zist Khamtir (Tehran, Iran). The second group received a placebo identically to its probiotic counterpart considering size, shape, color, weight, and package filled with starch. The groups of the study received the capsules in sealed envelopes and recommended storing them in the refrigerator, according to the product catalog, and consuming them twice daily with a full glass of water, for four months.
Outcome measures
The primary outcomes of the study included physical disability, cognitive function, depressive symptoms, and hand dexterity. Physical disability was assessed using the EDSS.23 EDSS was evaluated by an experienced neurologist, who was not aware of the received intervention. In addition, hand dexterity was measured using the Nine-Hole Peg Test (9HPT).24 All of the participants were right-handed and the tests were applied twice for both hands and the mean time for completing the test was recorded. The absolute values of the differences between the right and left hands were calculated and reported as asymmetry scores. Cognitive function was assessed using the Symbol Digit Modalities Test (SDMT),25 and the three-second version of the Paced Auditory Serial Addition Test (PASAT-3).26 Depressive symptoms were evaluated using the Persian-validated version27 of the Beck Depression Inventory-II (BDI-II).28 All assessments were conducted at baseline and in the second visit of the participants, after four months.
Sample size
Considering 80% power and type one error (α) of 0.05, allocation ratio 1, and based on the EDSS outcome in Kouchaki and colleagues’ RCT,29 the minimum sample size for this study was calculated using the G*Power software (version: 3.1.9.2) and 24 patients in each group was determined. Considering drop rates and in order to increase power, the sample size of 90 patients was determined for this study.
Randomization and blinding
A random allocation sequence was generated using a random number table, and 90 patients were randomized into intervention and placebo groups, so the type of randomization was simple. One researcher who did not participate in the data collection process enrolled participants and assigned them to groups. In order to double-blinding, in addition to the patients, the neurologist who assessed the clinical outcomes and the colleague who collected the data were not aware of the received intervention. The groups of the study received the capsules in sealed envelopes.
Data collection and statistical analysis
Data were collected at baseline and after the intervention through clinical examinations and standardized questionnaires. Data were analyzed using IBM SPSS Statistics for MacOS (Version 23). Descriptive statistics were used to summarize the data, with results presented as mean ± standard deviation or median [interquartile range] based on the normality of distributions assessed by the Shapiro-Wilk test. Between-group comparisons were performed using independent sample t-tests or the Mann-Whitney U test based on the normality of numeric distributions and Fisher’s Exact Test or chi-square for categorical variables. Paired sample t-test, Wilcoxon signed-rank test or McNemar’s test were used to evaluate the changes within groups. A p-value of less than 0.05 was considered statistically significant, and 95% confidence intervals were observed for all tests.
Results
A total of 122 patients were assessed for eligibility, of which 32 were excluded (18 did not meet inclusion criteria and 14 declined to participate). The remaining 90 patients were randomized into two groups: 45 were allocated to the probiotic group, and 45 to the placebo group. In the probiotic group, 42 patients received the allocated intervention, while three did not. In the placebo group, 43 patients received the allocated intervention. During the follow-up period, seven patients in the probiotic group were lost to follow-up, two patients discontinued the intervention due to disease relapse, and four patients discontinued the intervention due to gastrointestinal side effects and personal reasons. In the placebo group, 10 patients were lost to follow-up, and two discontinued the intervention (due to relapse and gastrointestinal side effects). Ultimately, 31 patients in the placebo group and 29 in the probiotic group completed the trial and were included in the final analysis. No patients were excluded from the final analysis. Constipation, was the only reported side effects in the participants, and there were no considerable safety issues in this trial. The flow of patient recruitment, allocation, follow-up, and analysis is presented in the CONSORT flow diagram (Figure 1).
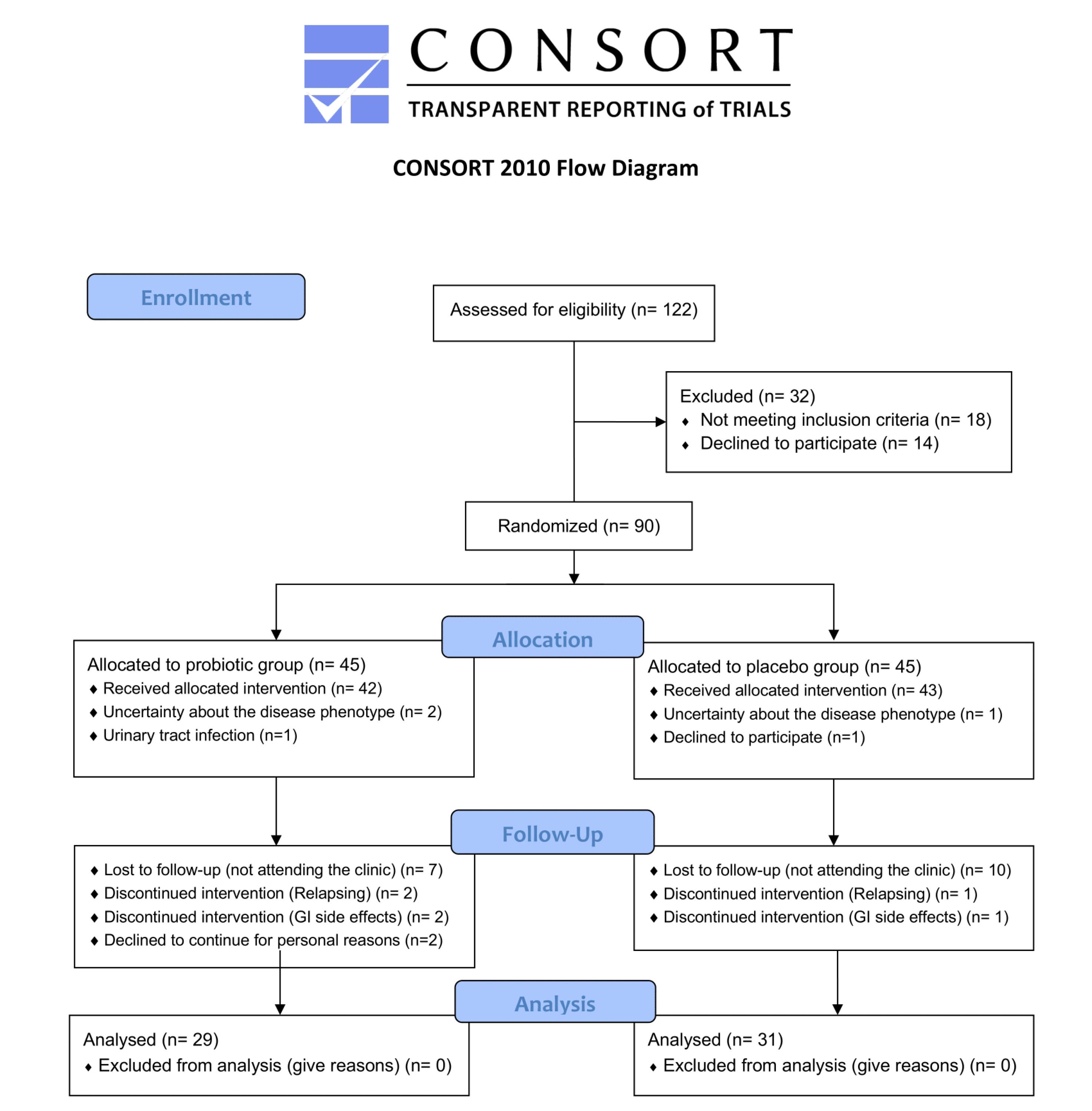
Figure 1.
CONSORT flow diagram
.
CONSORT flow diagram
Baseline characteristics
A total of 60 RRMS patients were included in the final analysis, with 29 in the probiotics group and 31 in the placebo group. The mean age was 33.66 ± 8.32 years for the probiotics group and 31.23 ± 8.85 years for the placebo group. The majority of patients in both groups were female (72.4% in the probiotics group and 80.6% in the placebo group). The duration of the disease was slightly longer in the probiotics group (60 months [IQR: 93]) compared to the placebo group (48 months [IQR: 63]). Details regarding the first clinical presentation, education levels, disease-modifying therapies, and smoking status are presented in Table 1.
Table 1.
Characteristics of the patients included in the final analysis
|
Characteristics (units, statistics)
|
Probiotics group (n=29)
|
Placebo group (n=31)
|
| Age (years-old, mean ± SD) |
33.66 ± 8.32 |
31.23 ± 8.85 |
| Female sex (n (percentage%)) |
21 (72.4%) |
25 (80.6%) |
| First presentation (n (percentage%)) |
|
|
| Ataxia |
3 (10.3%) |
2 (6.5%) |
| Blurred vision |
4 (13.8%) |
10 (32.3%) |
| Diplegia |
0 (0.0%) |
2 (6.5%) |
| Diplopia |
7 (24.1%) |
3 (9.7%) |
| Muscle Spasm |
0 (0.0%) |
1 (3.2%) |
| Optic Neuritis |
1 (3.4%) |
2 (6.5%) |
| Paresis |
2 (6.9%) |
0 (0.0%) |
| Sensory |
12 (41.4%) |
11 (35.5%) |
| Education (years, median [IQR]) |
14 [4] |
12 [5] |
| Disease duration (months, median [IQR]) |
60 [93] |
48 [63] |
| Disease-modifying therapy (n (percentage%)) |
|
|
| Dimethyl Fumarate |
7 (24.1%) |
14 (45.2%) |
| Fingolimod |
10 (34.5%) |
3 (9.7%) |
| Glatiramer acetate |
1 (3.4%) |
0 (0.0%) |
| Interferon-beta 1a |
6 (20.7%) |
4 (12.9%) |
| Natalizumab |
0 (0.0%) |
2 (6.5%) |
| Ocrelizumab |
1 (3.4%) |
4 (12.9%) |
| Rituximab |
3 (10.3%) |
3 (9.7%) |
| No disease-modifying therapy |
1 (3.4%) |
1 (3.2%) |
| Smoking |
2 (6.9%) |
2 (6.5%) |
SD: Standard deviation; IQR: Interquartile range.
Effects of probiotics supplementation in disability and functional scores
The EDSS did not show significant differences between the probiotics and placebo groups before or after the intervention (P > 0.05). Before treatment, the overall EDSS score was 0 for 48.3% of the probiotics group and 45.2% of the placebo group. After treatment, 51.7% of patients in the probiotics group and 51.6% in the placebo group had an overall EDSS score of 0 (P = 0.35). No significant changes were observed within either group (P = 0.30 for the probiotics group, P = 0.40 for the placebo group) (Table 2).
Table 2.
The effects of probiotic supplementation on the level of disability, based on the Expanded Disability Status Scale (EDSS).
|
Scale (timing, statistics)
|
Probiotics group (n=29)
|
Placebo group (n=31)
|
Between groups
P
value
|
EDSS overall score
(Before, n (percentage%)) |
0 |
14 (48.3%) |
14 (45.2%) |
0.44 |
| 1 |
8 (27.6%) |
11 (35.5%) |
| 1.5 |
3 (10.3%) |
1 (3.2%) |
| 2 |
1 (3.4%) |
4 (12.9%) |
| 3 |
2 (6.9%) |
0 (0.0%) |
| 3.5 |
1 (3.4%) |
1 (3.2%) |
| EDSS overall score (Before, median [IQR]) |
1 [1.25] |
1.0 [1.0] |
0.99 |
EDSS overall score
(After, n (percentage%)) |
0 |
15 (51.7%) |
16 (51.6%) |
0.49 |
| 1 |
9 (31.0%) |
8 (25.8%) |
| 1.5 |
1 (3.4%) |
1 (3.2%) |
| 2 |
1 (3.4%) |
5 (16.1%) |
| 3 |
2 (6.9%) |
0 (0.0%) |
| 3.5 |
1 (3.4%) |
1 (3.2%) |
| EDSS overall score (After, median [IQR]) |
0.0 [1.0] |
0.0 [1.0] |
0.91 |
| Intragroup comparison of EDSS scores (P value) |
0.30 |
0.40 |
- |
EDSS: Expanded Disability Status Scale; IQR: Interquartile range.
Effects of probiotics supplementation on depressive symptoms
Depressive symptoms, based on BDI-II scores, showed significant improvement in the placebo group (P = 0.02). The median change in BDI-II scores was 0 [IQR: 9] for the probiotics group and -3 [IQR: 6] for the placebo group (P = 0.11). Nor before (P = 0.23), nor after the interventions (P = 0.10), there was no significant difference between the groups of the study regarding the depressive symptoms (Table 3).
Table 3.
The effects of probiotic supplementation on depressive symptoms, based on the second version of Beck’s Depression Inventory (BDI-II)
|
Scale (timing, statistics)
|
Probiotics group (n=29)
|
Placebo group (n=31)
|
Between groups
P
value
|
| BDI-II scores (before, median [IQR]) |
12 [13] |
13 [19] |
0.48 |
| BDI-II scores (after, median [IQR]) |
13 [16] |
9 [17] |
0.79 |
| BDI-II scores (changes, median [IQR]) |
0 [9] |
-3 [6] |
0.11 |
| Intragroup comparison of BDI-II scores (P value) |
0.86 |
0.02* |
- |
| Depressive symptoms (Before, n (percentage%)) |
Minimal (BDI-II < 13) |
16 (55.2%) |
16 (51.6%) |
0.23 |
| Mild (BDI-II: 14-19) |
7 (24.1%) |
3 (9.7%) |
| Moderate (BDI-II: 20-28) |
2 (6.9%) |
7 (22.6%) |
| Severe (BDI-II > 28) |
4 (13.8%) |
5 (16.1%) |
| Depressive symptoms (After, n (percentage%)) |
Minimal (BDI-II < 13) |
15 (51.7%) |
19 (61.3%) |
0.10 |
| Mild (BDI-II: 14-19) |
7 (24.1%) |
2 (6.5%) |
| Moderate (BDI-II: 20-28) |
2 (6.9%) |
7 (22.6%) |
| Severe (BDI-II > 28) |
5 (17.2%) |
3 (9.7%) |
| Intragroup comparison of depressive symptoms (P value) |
0.70 |
0.33 |
- |
BDI-II: second version of Beck’s Depression Inventory; IQR: Interquartile range. * Statistically significant
Effects of Probiotics Supplementation on Cognitive Function
Cognitive function, assessed through SDMT and PASAT-3, revealed no significant differences between the groups. The mean change in SDMT scores was 0.5 [IQR: 6] in the probiotics group and 0 [IQR: 9] in the placebo group (P = 0.96). Similarly, PASAT-3 scores showed non-significant changes between the groups (P = 0.76). However, both groups demonstrated significant improvements in PASAT-3 scores after treatment (P = 0.02 for the probiotics group, P = 0.01 for the placebo group) (Table 4).
Table 4.
The effects of probiotic supplementation on cognitive function, based on the Symbol Digit Modalities Test (SDMT) and the three-second version of the Paced Auditory Serial Addition Test (PASAT-3)
|
Scale (timing, statistics)
|
Probiotics group (n=29)
|
Placebo group (n=31)
|
Between groups
P
value
|
| SDMT scores (before, mean ± SD) |
47.08 ± 12.24 |
49.90 ± 13.16 |
0.47 |
| SDMT scores (after, mean ± SD) |
48.79 ± 12.01 |
50.62 ± 15.85 |
0.55 |
| SDMT scores (changes, median [IQR]) |
0.5 [6] |
0 [9] |
0.96 |
| Intragroup comparison of SDMT scores (P value) |
0.62 |
0.70 |
- |
| SDMT impairment (before SDMT < 30.86), n (percentage%) |
6 (20.7%) |
2 (6.5%) |
0.14 |
| SDMT impairment (after SDMT < 30.86), n (percentage%) |
4 (13.8%) |
5 (16.1%) |
0.99 |
| Intragroup comparison of SDMT impairment (P value) |
0.49 |
0.19 |
- |
| PASAT-3 scores (before, mean ± SD) |
49.58 ± 8.36 |
45.45 ± 9.17 |
0.09 |
| PASAT-3 scores (after, mean ± SD) |
52.96 ± 7.27 |
49.00 ± 8.33 |
0.07 |
| PASAT-3 scores (changes, median [IQR]) |
2 [9.5] |
2 [6] |
0.76 |
| Intragroup comparison of PASAT-3 scores (P value) |
0.02* |
0.01* |
- |
| PASAT-3 impairment (before PASAT-3 < 33.71), n (percentage%) |
7 (24.1%) |
4 (12.9%) |
0.33 |
| PASAT-3 impairment (after PASAT-3 < 33.71), n (percentage%) |
5 (17.2%) |
3 (9.7%) |
0.46 |
| Intragroup comparison of PASAT-3 impairment (P value) |
0.52 |
0.70 |
- |
SDMT: Symbol Digit Modalities Test; PASAT-3: three seconds version of Paced Auditory Serial Addition Test; IQR: Interquartile range; SD: Standard deviation.
Note: Impairment was defined as ≤ − 1.5 standard deviations from the mean normative values for each cognitive test in the Iranian general population.
* Statistically significant
Effects of probiotics supplementation on hand dexterity
Hand dexterity, measured using 9HPT, indicated the mean 9HPT score for the dominant hand improved significantly in both the probiotics (P = 0.04) and the placebo group (P = 0.01), with no significant differences between groups (P = 0.43). Left-hand (non-dominant) dexterity as well as the 9HPT mean scores, showed significant improvement only in the placebo group (P < 0.01). Asymmetry between the hands in 9HPT did not show significant changes in either group (Table 5).
Table 5.
The effects of probiotic supplementation on hand dexterity, based on the Nine-Hole Peg Test (9HPT)
|
Scale (timing, statistics)
|
Probiotics group (n=29)
|
Placebo group (n=31)
|
Between groups
P
value
|
| 9HPT-R scores (before, mean ± SD) |
22.16 ± 2.19 |
24.18 ± 4.44 |
0.03 * |
| 9HPT-R scores (after, mean ± SD) |
21.08 ± 2.23 |
22.98 ± 4.70 |
0.05 |
| 9HPT-R scores (changes, median [IQR]) |
-0.43 [2.15] |
-1.03 [3.29] |
0.43 |
| Intragroup comparison of 9HPT-R scores (P value) |
0.04* |
0.01* |
- |
| 9HPT-L scores (before, mean ± SD) |
23.43 ± 2.95 |
25.88 ± 3.96 |
0.01* |
| 9HPT-L scores (after, mean ± SD) |
23.44 ± 3.15 |
24.65 ± 4.67 |
0.24 |
| 9HPT-L scores (changes, median [IQR]) |
0 [1.75] |
-1.13 [3.71] |
0.07 |
| Intragroup comparison of 9HPT-L scores (P value) |
0.97 |
< 0.01* |
- |
| 9HPT-M scores (before, median [IQR]) |
22.79 ± 2.37 |
25.02 ± 4.05 |
0.01* |
| 9HPT-M scores (after, median [IQR]) |
22.26 ± 2.46 |
23.81 ± 4.45 |
0.09 |
| 9HPT-M scores (changes, median [IQR]) |
0.00 [1.73] |
-0.69 [2.32] |
0.11 |
| Intragroup comparison of 9HPT-M scores (P value) |
0.11 |
< 0.01* |
- |
| 9HPT-A scores (before, median [IQR]) |
1.42 [1.70] |
1.57 [3.51] |
0.53 |
| 9HPT-A scores (after, median [IQR]) |
2.18 [2.96] |
2.25 [2.99] |
0.97 |
| 9HPT-A scores (changes, median [IQR]) |
0.14 [2.03] |
0.34 [2.03] |
0.92 |
| Intragroup comparison of 9HPT-A scores (P value) |
0.06 |
0.15 |
- |
9HPT-R: Nine-Hole Peg Test, right hand; 9HPT-L: Nine-Hole Peg Test, left hand; 9HPT-M: Nine-Hole Peg Test, mean; 9HPT-A: Nine-Hole Peg Test, asymmetry; IQR: Interquartile range; SD: Standard deviation. * Statistically significant
Discussion
This RCT explored the effects of probiotics on level of disability, cognitive performance, depressive symptoms, and hand dexterity in RRMS patients. Overall, the findings of this RCT revealed no significant improvement in the mentioned factors, with four-month supplementation with a seven-strain probiotic (Lactocare®) in RRMS patients with EDSS < 4. In comparison, previously, a limited number of studies have shown more promising results regarding the clinical improvements in MS symptoms by reducing inflammatory and oxidative biomarkers.30,31 Although this study did not focus on inflammatory biomarkers, these findings suggest that probiotics may have a broader range of benefits beyond the measured outcomes, potentially improving clinical symptoms through immune modulation.32 The findings of this RCT show some improvements in hand function and cognitive outcomes, but the overall effects were modest.
In terms of depressive symptoms, the present study found no substantial improvement with probiotics supplementation. A recent meta-analysis, based on the results of three RCTs, suggested the positive effects of probiotics in improving the depressive symptoms associated with MS.33 In detail, Rahimlou et al, in an RCT of 70 patients with RRMS patients based on 2005 revised McDonald criteria, with EDSS ≤ 4.5, found that six-month administration of 14 strains of probiotic supplementation, was effective in boosting depressive symptoms. The baseline BDI scores in this study were 22.15 ± 1.62 and 20.84 ± 1.25 in the intervention and placebo groups.32 Salami et al, in an evaluation of 48 RRMS patients, with EDSS ≤ 4.5, found daily intake of Lactocare® supplementation for 16 weeks, effective in improving the depressive symptoms in patients with baseline BDI scores of 18.2 ± 1.52 and 21.12 ± 1.35, in the placebo and probiotics groups, respectively.31 In Kouchaki and colleagues’ RCT of 54 RRMS patients with EDSS ≤ 4.5, daily supplementation with a probiotic product containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum, for 12 weeks, changes in BDI scores in were significantly different between the placebo and intervention groups; however, the baseline BDI scores were not reported in this study and there was no exclusion of patients with diagnosed depression in this trial.29 In the present RCT, the median baseline BDI scores were 12 [IQR: 13] and 13 [IQR: 19] in the probiotics and placebo groups, respectively. Considering the evidence regarding the positive effects of probiotics in patients with clinical diagnosis of depression,34 minimal depressive symptoms in the participants as well as exclusion of patients with diagnosed clinical depression in this study, may be the cause of non-significant findings in this regard. Additionally, the interaction between gut microbiota and depressive function may differ depending on individual gut compositions or the degree of disease progression in MS patients. The mentioned three studies also suggested probiotic supplementation was effective in improving the EDSS scores of the participants. Similar to the present RCT, all of the mentioned studies only included RRMS patients with EDSS ≤ 4.5. The presence of dysbiosis in MS patients has been associated with increased physical disability, and correcting this imbalance with probiotics could offer some physical benefits35; however, the lack of improvements in depressive and physical symptoms in our probiotics group may reflect variations in individual responses or differences in probiotic strain or dosage. In addition, differences in specific strains, doses of probiotics, duration of supplementation used, and MS diagnostic criteria - which was the 2017 revised version of McDonald criteria, may contribute to this disagreement, too.
Cognitive dysfunction is suggested as a poorly managed symptom of MS.36 Probiotics are suggested to enhance cognitive performance in MS through the anti-inflammation pathway.37 Evidence suggested positive effects of probiotics in improving the cognitive function of patients with mild cognitive impairment and/or Alzheimer’s disease,38 but not in the elderly.39 To the best of our knowledge, there was no previous study that investigated the cognitive effects of probiotics in MS.40 The findings of this study revealed no significant improvement with probiotics supplementation after the four-month intervention. Specifically, SDMT scores, as a well-known test for evaluation of visual information process speed, showed minimal improvement in both groups, with no changes observed between groups. While both groups exhibited improvements on PASAT-3, which is suggested as a standard test for auditory information process speed, the between-group differences were not observed. This study did not support the observed cognitive improvement in animal models of MS; which may be due to the short duration of the supplementation, too.
With regard to manual dexterity, the probiotics supplementation was not found to improve hand dexterity, as measured by 9HPT, which is considered a gold standard measure of manual dexterity in MS.41 Both groups demonstrated improvements in dominant-hand functioning, but the between-group differences were not observed. In addition, 9HPT mean and asymmetry scores which are suggested as precise assessment of hand functioning,24,42 are not found to be affected by probiotic supplementation in this study. Best of our knowledge, there were no previous studies that assessed these effects and they should be further researched, considering the positive effects of this supplementation in improving muscle strength and functional performance in other conditions such as chronic obstructive pulmonary disease,43 and sarcopenia.44
Despite the considerable strengths of the present RCT such as double-blinded study design, cognitive assessments, and investigating the manual dexterity; as a single-center RCT, the main limitation of this study was the limited sample size, which is suggested to be addressed in future studies. Evaluation of the longer-term effects of probiotic supplementation is also recommended. Multiple factors including poly-symptomatic presentation, longer duration of MS attacks, smoking, body mass index (BMI),45 and onset of the disease in ages > 50,46 are factors associated with MS progression which may affect the findings of this study as confounders. The generalizability of the findings of this study is limited to RRMS patients with EDSS < 4; therefore, it cannot be extended to severely disabled patients and/or patients with progressive forms of the disease. In addition, homogeneity regarding the ethnicity and setting of the study which was only one clinic in Tabriz, Iran may affect the external validity of the findings of this study.
Conclusion
This RCT found that supplementation with a seven-strain probiotics product for four months did not result in a significant improvement in the level of disability, depressive symptoms, cognitive performance, and manual dexterity of RRMS patients with EDSS < 4.
Competing Interests
None declared.
Data Availability Statement
The dataset analyzed during the current study is available from the corresponding author on a reasonable request.
Ethical Approval
The study protocol was approved by the ethics committee of Tabriz University of Medical Sciences (ethical approval code: IR.TBZMED.REC.1401.426, approval date: 2022.08.01), and informed consent was obtained from all participants. The trial was registered with the Iranian Registry of Clinical Trials (IRCT20220713055465N1, registration date: 2022.08.30).
Acknowledgements
We would like to appreciate the cooperation of the Clinical Research Development Unit, Imam Reza General Hospital, Tabriz, Iran in conducting this research.
References
- Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol 2019; 26(1):27-40. doi: 10.1111/ene.13819 [Crossref] [ Google Scholar]
- Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler 2020; 26(14):1816-21. doi: 10.1177/1352458520970841 [Crossref] [ Google Scholar]
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17(2):162-73. doi: 10.1016/s1474-4422(17)30470-2 [Crossref] [ Google Scholar]
- Benedict RH, Amato MP, DeLuca J, Geurts JJ. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol 2020; 19(10):860-71. doi: 10.1016/s1474-4422(20)30277-5 [Crossref] [ Google Scholar]
- De Meo E, Portaccio E, Giorgio A, Ruano L, Goretti B, Niccolai C. Identifying the distinct cognitive phenotypes in multiple sclerosis. JAMA Neurol 2021; 78(4):414-25. doi: 10.1001/jamaneurol.2020.4920 [Crossref] [ Google Scholar]
- Oset M, Stasiolek M, Matysiak M. Cognitive dysfunction in the early stages of multiple sclerosis-how much and how important?. Curr Neurol Neurosci Rep 2020; 20(7):22. doi: 10.1007/s11910-020-01045-3 [Crossref] [ Google Scholar]
- Lublin FD, Häring DA, Ganjgahi H, Ocampo A, Hatami F, Čuklina J. How patients with multiple sclerosis acquire disability. Brain 2022; 145(9):3147-61. doi: 10.1093/brain/awac016 [Crossref] [ Google Scholar]
- Stuart CM, Varatharaj A, Domjan J, Philip S, Galea I. Physical activity monitoring to assess disability progression in multiple sclerosis. Mult Scler J Exp Transl Clin 2020; 6(4):2055217320975185. doi: 10.1177/2055217320975185 [Crossref] [ Google Scholar]
- Renner A, Baetge SJ, Filser M, Penner IK. Working ability in individuals with different disease courses of multiple sclerosis: factors beyond physical impairment. Mult Scler Relat Disord 2020; 46:102559. doi: 10.1016/j.msard.2020.102559 [Crossref] [ Google Scholar]
- Parodi B, Kerlero de Rosbo N. The gut-brain axis in multiple sclerosis. Is its dysfunction a pathological trigger or a consequence of the disease? Front Immunol 2021; 12:718220. doi: 10.3389/fimmu.2021.718220 [Crossref] [ Google Scholar]
- Kumar N, Sahoo NK, Mehan S, Verma B. The importance of gut-brain axis and use of probiotics as a treatment strategy for multiple sclerosis. Mult Scler Relat Disord 2023; 71:104547. doi: 10.1016/j.msard.2023.104547 [Crossref] [ Google Scholar]
- Eastwood J, Walton G, Van Hemert S, Williams C, Lamport D. The effect of probiotics on cognitive function across the human lifespan: a systematic review. Neurosci Biobehav Rev 2021; 128:311-27. doi: 10.1016/j.neubiorev.2021.06.032 [Crossref] [ Google Scholar]
- Chao L, Liu C, Sutthawongwadee S, Li Y, Lv W, Chen W. Effects of probiotics on depressive or anxiety variables in healthy participants under stress conditions or with a depressive or anxiety diagnosis: a meta-analysis of randomized controlled trials. Front Neurol 2020; 11:421. doi: 10.3389/fneur.2020.00421 [Crossref] [ Google Scholar]
- Akhgarjand C, Vahabi Z, Shab-Bidar S, Etesam F, Djafarian K. Effects of probiotic supplements on cognition, anxiety, and physical activity in subjects with mild and moderate Alzheimer’s disease: a randomized, double-blind, and placebo-controlled study. Front Aging Neurosci 2022; 14:1032494. doi: 10.3389/fnagi.2022.1032494 [Crossref] [ Google Scholar]
- Khatun M, Hoque F, Hoque NS, Hossain MS, Alam MA, Afrin S. Emerging role of probiotics in advancement of combating physical abnormalities and diseases: a systematic perspective analysis. Asian J Biochem Genet Mol Biol 2024; 16(8):1-23. doi: 10.9734/ajbgmb/2024/v16i8397 [Crossref] [ Google Scholar]
- Brown J, Quattrochi B, Everett C, Hong BY, Cervantes J. Gut commensals, dysbiosis, and immune response imbalance in the pathogenesis of multiple sclerosis. Mult Scler 2021; 27(6):807-11. doi: 10.1177/1352458520928301 [Crossref] [ Google Scholar]
- Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther 2015; 37(5):984-95. doi: 10.1016/j.clinthera.2015.04.002 [Crossref] [ Google Scholar]
- Lalonde R, Strazielle C. Probiotic influences on motor skills: a review. Curr Neuropharmacol 2023; 21(12):2481-6. doi: 10.2174/1570159x21666230807150523 [Crossref] [ Google Scholar]
- Dziedzic A, Saluk J. Probiotics and commensal gut microbiota as the effective alternative therapy for multiple sclerosis patients treatment. Int J Mol Sci 2022; 23(22):14478. doi: 10.3390/ijms232214478 [Crossref] [ Google Scholar]
- Ghadiri F, Ebadi Z, Asadollahzadeh E, Naser Moghadasi A. Gut microbiome in multiple sclerosis-related cognitive impairment. Mult Scler Relat Disord 2022; 67:104165. doi: 10.1016/j.msard.2022.104165 [Crossref] [ Google Scholar]
- Jiang J, Chu C, Wu C, Wang C, Zhang C, Li T. Efficacy of probiotics in multiple sclerosis: a systematic review of preclinical trials and meta-analysis of randomized controlled trials. Food Funct 2021; 12(6):2354-77. doi: 10.1039/d0fo03203d [Crossref] [ Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010; 8:18. doi: 10.1186/1741-7015-8-18 [Crossref] [ Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33(11):1444-52. doi: 10.1212/wnl.33.11.1444 [Crossref] [ Google Scholar]
- Solaro C, Grange E, Di Giovanni R, Cattaneo D, Bertoni R, Prosperini L. Nine Hole Peg Test asymmetry in refining upper limb assessment in multiple sclerosis. Mult Scler Relat Disord 2020; 45:102422. doi: 10.1016/j.msard.2020.102422 [Crossref] [ Google Scholar]
- Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler 2017; 23(5):721-33. doi: 10.1177/1352458517690821 [Crossref] [ Google Scholar]
- Wiens AN, Fuller KH, Crossen JR. Paced Auditory Serial Addition Test: adult norms and moderator variables. J Clin Exp Neuropsychol 1997; 19(4):473-83. doi: 10.1080/01688639708403737 [Crossref] [ Google Scholar]
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N. Psychometric properties of a Persian-language version of the Beck Depression Inventory--second edition: BDI-II-PERSIAN. Depress Anxiety 2005; 21(4):185-92. doi: 10.1002/da.20070 [Crossref] [ Google Scholar]
- Beck AT, Steer RA, Brown G. Beck Depression Inventory–II. [Database record]. APA PsycTests; 1996. doi: 10.1037/t00742-000.
- Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr 2017; 36(5):1245-9. doi: 10.1016/j.clnu.2016.08.015 [Crossref] [ Google Scholar]
- Motlagh Asghari K, Dolatkhah N, Ayromlou H, Mirnasiri F, Dadfar T, Hashemian M. The effect of probiotic supplementation on the clinical and para-clinical findings of multiple sclerosis: a randomized clinical trial. Sci Rep 2023; 13(1):18577. doi: 10.1038/s41598-023-46047-6 [Crossref] [ Google Scholar]
- Salami M, Kouchaki E, Asemi Z, Tamtaji OR. How probiotic bacteria influence the motor and mental behaviors as well as immunological and oxidative biomarkers in multiple sclerosis? A double-blind clinical trial. J Funct Foods 2019; 52:8-13. doi: 10.1016/j.jff.2018.10.023 [Crossref] [ Google Scholar]
- Rahimlou M, Hosseini SA, Majdinasab N, Haghighizadeh MH, Husain D. Effects of long-term administration of Multi-Strain Probiotic on circulating levels of BDNF, NGF, IL-6 and mental health in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Nutr Neurosci 2022; 25(2):411-22. doi: 10.1080/1028415x.2020.1758887 [Crossref] [ Google Scholar]
- Mirashrafi S, Hejazi Taghanaki SZ, Sarlak F, Moravejolahkami AR, Hojjati Kermani MA, Haratian M. Effect of probiotics supplementation on disease progression, depression, general health, and anthropometric measurements in relapsing-remitting multiple sclerosis patients: a systematic review and meta-analysis of clinical trials. Int J Clin Pract 2021; 75(11):e14724. doi: 10.1111/ijcp.14724 [Crossref] [ Google Scholar]
- Zhang Q, Chen B, Zhang J, Dong J, Ma J, Zhang Y. Effect of prebiotics, probiotics, synbiotics on depression: results from a meta-analysis. BMC Psychiatry 2023; 23(1):477. doi: 10.1186/s12888-023-04963-x [Crossref] [ Google Scholar]
- Pellizoni FP, Leite AZ, de Campos Rodrigues N, Ubaiz MJ, Gonzaga MI, Takaoka NNC. Detection of dysbiosis and increased intestinal permeability in Brazilian patients with relapsing-remitting multiple sclerosis. Int J Environ Res Public Health 2021; 18(9):4621. doi: 10.3390/ijerph18094621 [Crossref] [ Google Scholar]
- Motavalli A, Majdi A, Hosseini L, Talebi M, Mahmoudi J, Hosseini SH. Pharmacotherapy in multiple sclerosis-induced cognitive impairment: a systematic review and meta-analysis. Mult Scler Relat Disord 2020; 46:102478. doi: 10.1016/j.msard.2020.102478 [Crossref] [ Google Scholar]
- Jiang J, Chu C, Wu C, Wang C, Zhang C, Li T. Efficacy of probiotics in multiple sclerosis: a systematic review of preclinical trials and meta-analysis of randomized controlled trials. Food Funct 2021; 12(6):2354-77. doi: 10.1039/d0fo03203d [Crossref] [ Google Scholar]
- Mo R, Jiang M, Xu H, Jia R. Effect of probiotics on cognitive function in adults with mild cognitive impairment or Alzheimer’s disease: a meta-analysis of randomized controlled trials. Med Clin (Barc) 2024; 162(12):565-73. doi: 10.1016/j.medcli.2024.01.013 [Crossref] [ Google Scholar]
- Tahmasbi F, Mirghafourvand M, Shamekh A, Mahmoodpoor A, Sanaie S. Effects of probiotic supplementation on cognitive function in elderly: a systematic review and meta-analysis. Aging Ment Health 2022; 26(9):1778-86. doi: 10.1080/13607863.2021.1966743 [Crossref] [ Google Scholar]
- Tsogka A, Kitsos DK, Stavrogianni K, Giannopapas V, Chasiotis A, Christouli N. Modulating the gut microbiome in multiple sclerosis management: a systematic review of current interventions. J Clin Med 2023; 12(24):7610. doi: 10.3390/jcm12247610 [Crossref] [ Google Scholar]
- Feys P, Lamers I, Francis G, Benedict R, Phillips G, LaRocca N. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult Scler 2017; 23(5):711-20. doi: 10.1177/1352458517690824 [Crossref] [ Google Scholar]
- Koch MW, Repovic P, Mostert J, Bowen JD, Comtois J, Strijbis E. The nine hole peg test as an outcome measure in progressive MS trials. Mult Scler Relat Disord 2023; 69:104433. doi: 10.1016/j.msard.2022.104433 [Crossref] [ Google Scholar]
- Karim A, Muhammad T, Shahid Iqbal M, Qaisar R. A multistrain probiotic improves handgrip strength and functional capacity in patients with COPD: a randomized controlled trial. Arch Gerontol Geriatr 2022; 102:104721. doi: 10.1016/j.archger.2022.104721 [Crossref] [ Google Scholar]
- Qaisar R, Burki A, Karim A, Shahid Iqbal M, Ahmad F. Probiotics supplements improve the sarcopenia-related quality of life in older adults with age-related muscle decline. Calcif Tissue Int 2024; 114(6):583-91. doi: 10.1007/s00223-024-01211-6 [Crossref] [ Google Scholar]
- Hosny HS, Shehata HS, Ahmed S, Ramadan I, Abdo SS, Fouad AM. Predictors of severity and outcome of multiple sclerosis relapses. BMC Neurol 2023; 23(1):67. doi: 10.1186/s12883-023-03109-6 [Crossref] [ Google Scholar]
- Naseri A, Nasiri E, Sahraian MA, Daneshvar S, Talebi M. Clinical features of late-onset multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord 2021; 50:102816. doi: 10.1016/j.msard.2021.102816 [Crossref] [ Google Scholar]