Pharmaceutical Sciences. 2025;31(3):288-293.
doi: 10.34172/PS.025.40963
Research Article
Isolation and Characterization of Bioactive Compounds from Scotch Thistle (Onopordum acanthium L.) Seeds
Sakineh Mohammadi Formal analysis, Investigation, Writing – original draft, 1 
Ali Movafeghi Validation, Writing – review & editing, 1
Abbas Delazar Methodology, Writing – review & editing, 2
Sanaz Hamedeyazdan Visualization, Writing – review & editing, 2 
Mir Babak Bahadori Investigation, Visualization, Writing – original draft, 3, * 
Hossein Nazemiyeh Conceptualization, Methodology, Supervision, Writing – review & editing, 4, * 
Author information:
1Department of Plant Biology, Faculty of Natural Sciences, University of Tabriz, Tabriz, Iran
2Department of Pharmacognosy, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
3Medicinal Plants Research Center, Maragheh University of Medical Sciences, Maragheh, Iran
4Research Center for Pharmaceutical Nanotechnology, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Scotch thistle (Onopordum acanthium L.) is a medicinal food plant known for its phytochemical diversity.
Methods:
In this study, methanolic and petroleum ether extracts of Scotch thistle seeds were subjected to phytochemical analysis. As regards, methanol extract was fractionated via different chromatographic methods and structures of the isolated pure compounds were elucidated with relative nuclear magnetic resonance spectra. Additionally, scotch thistle oil was analyzed via gas chromatography-mass spectrometry (GC-MS). Besides, the in vitro 2,2-diphenyl-1-picrylhydrazyl (DPPH) antioxidant property of O. acanthium extract was evaluated.
Results:
Structure elucidation of the isolated major compounds of the extract yielded to seven major metabolites, revealing the presence of five lignan and two serotonin derivatives, including arctiin, arctigenin, and matairesinol, with six compounds being newly reported from O. acanthium. Furthermore, GC-MS analysis identified eight fatty acids in the seed oil, with linoleic acid (63.4%) and 13-octadecenoic acid (28.2%) being the predominant components. The antioxidant potential of scotch thistle exhibited strong antioxidant activity, as reflected by RC50 values ranging from 2.1 to 501.9 µg/mL for various methanolic fractions of O. acanthium.
Conclusion:
These findings highlighted the rich bioactive content of scotch thistle, underscoring its significance in medicinal and ecological contexts.
Keywords: Arctiin, Arctigenin, Matairesinol, Lignan, Serotonin, Thistle
Copyright and License Information
© 2025 The Author(s).
This is an open access article and applies the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
None.
Introduction
Onopordum spp. are known to thrive in various habitats across Europe, the Middle East, the Caucasus, and Central Asia. Among the species within this genus, Onopordum acanthium L., commonly referred to as Scotch thistle, stands out as a biennial plant indigenous to Western Asia and Europe. The genus Onopordum, a member of the Asteraceae family, is comprised of 50 species distributed worldwide, with 7 of these species being identified in the flora of Iran, 5 of which are endemic to the region.1 Onopordum spp. are known to thrive in various habitats across Europe, the Middle East, the Caucasus, and Central Asia. Among the species within this genus, O. acanthium L., commonly referred to as Scotch thistle, stands out as a biennial plant indigenous to Western Asia and Europe.2 Traditional applications of Scotch thistle encompass a wide array of medicinal uses, including its utilization as a natural antimicrobial, hypotensive, anticancer, cardiotonic, diuretic, anti-inflammatory, and hemostatic agent.3,4 Recent scientific studies have further unveiled the diverse pharmacological properties of the plant, demonstrating its anti-inflammatory, antihypertensive, antibacterial,5 and antioxidant capabilities, as well as its potential for augmenting natural killer cell activity against tumor cells.2,4,6 Phytochemical analysis of O. acanthium has divulged the presence of various natural compounds, including flavonoids, phenolic acids, amino acids, steroids, triterpenoids, coumarins, and acetylenic compounds.1,7,8 Of particular interest is the significant abundance of lignans found in Onopordum spp., serving as crucial defense mechanisms against pathogens. Lignans play pivotal roles in chemical defense functions, exhibiting activities including fungicidal, bactericidal, and insecticidal properties, while also displaying biological effects such as antiviral, antidiabetic, anticancer, and trypanocidal activities.9-12 Furthermore, some lignans function as allelopathic agents, contributing to the regulation and growth modulation of surrounding plant species.13,14 Lignans are involved in the regulation and modulation of growth. Dispersion of lignans in most plant families suggests their roles in growth and evolution. In the ecologic level, lignans possess critical roles in interactions of plants with other organisms which is mostly due to their defensive properties and in the protection against physical damages.
Given the extensive traditional uses, pharmacological activities, wide distribution, and industrial applications of Scotch thistle, we aimed to investigate the phytoconstituents and antioxidant potential of O. acanthium L. seeds, collected from the Azerbaijan province, Iran. Through our research, we seek to elucidate the bioactive compounds present in Scotch thistle and explore their potential health benefits and ecological significance.
Methods
Plant material
O. acanthium L. seeds were collected in late August 2018 from Maragheh-Hashtroud and Maragheh-Ajabshir roads, Eastern Azerbaijan Province, Iran. A voucher specimen (TBZFPH539) after authentication of the plant was deposited at the herbarium of the Faculty of Pharmacy, Tabriz University of Medical Science, Iran. Seeds were dried in dark, and powdered using a blender in order for solvent extraction.
Solvent extraction, fractionation and isolation
Dried and ground seeds of O. acanthium L. (200 g) were extracted using soxhlet apparatus successively with 1L of petroleum ether, dichloromethane and methanol, separately. All extracts one-to-one were concentrated and dried completely using a rotary evaporator (Heidolph, Germany) at 40 ºC in vacuum, yielding 61.1, 2.0, and 35.9 g dried extracts, respectively. Crude extracts were stored in tight containers in the dark and refrigerator until analysis. The extracts were preliminary monitored with analytical thin layer chromatography (TLC) to quality check the presence of phytochemicals groups within the extracts (TLC plates with 0.2 mm silica gel GF254). Accordingly, the methanol extract rich in different groups of phytochemicals was selected for further analysis. In order for methanol extract fractionation, solid phase extraction (SPE) method was carried out via reversed phase Sep-pak cartridge (C18, 10 g, Waters, Ireland). Two grams of the methanol extract was suspended in 10 mL of water/methanol (80:20). The SPE column was eluted using 200 mL of 20:80, 40:60, 60:40, 80:20 and 100:0 of methanol/water in step gradients, respectively. This process of fractionation was repeated three times under vacuum conditions and the flow rate of the solvent mixture was 8-15 drop/min. All resultant fractions were collected and dried using a rotary evaporator at 40 ºC in vacuum. All the 5 obtained fractions were weighed, and then kept in tight vials in the refrigerator for subsequent analyses. Preliminary analysis of the methanol fractions was fulfilled applying TLC analysis. It was shown that the two 40% and 60% SPE fractions covered the prime major compounds which was subjected to reverse phase high performance liquid chromatography (RP-HPLC) analysis for further fractionation to achieve the topmost components of the O. acanthium methanol extract. As regards, HPLC Shimadzu apparatus was utilized with an ODS Dr. Maisch Gmbh reprosil 100 preparative column (10 μm, 250 × 4.6 mm, 5 µm), UV-PDA as the detector. Separations for 40% SPE fraction were succeeded through a linear gradient of mobile phase of 25-40% methanol in water; 60 minutes; flow rate: 10 ml.min-1, afforded 2 compounds with retention time of 10.9 minutes (compound I) and 52.0min (compound II) were collected according to the HPLC chromatogram. Likewise, separations for 60% SPE fraction were succeeded through a linear gradient of mobile phase of 50%-60% methanol in water; 60 minutes; flow rate: 10 mL.min-1, afforded 5 compounds with retention time of 5.5 minutes (compound III), 6.0 minutes (compound IV), 8.7 minutes (compound V), 12 minutes (compound VI) and 27 minutes (compound VII) were collected according to the HPLC chromatogram. Structure elucidation of the purified compounds was accomplished with NMR spectra that were recorded on a Bruker 400 MHz spectrometer operating at 400 MHz for 1H and 100 MHz for 13C. Methanol-d4 was used for NMR analysis.
Esterification and analysis of fatty acids
Preparation of fatty acid methyl ester (FAME) derivatives of petroleum ether extract was carried out using a simple and fast procedure. Briefly, 10 mg of petroleum ether extract was placed into a small glass tube with 2 mL n-hexane and 0.2 mL methanolic KOH (2 M). Afterward, the tube was vortexed for 2 min at room temperature for methanolysis reaction. An aliquot of the upper layer (n-hexane) of mixture in the reaction tube was directly injected into gas chromatography mass spectrometry (GC-MS) for further analysis of the components. Consequently, GC-MS (Shimadzu, Japan, QP-5050 A) on a DB-1 capillary column (60 m length, 0.25 mm i.d., 0.25 μm stationary thickness) was utilized coupled with an electron impact (EI) ionization system in which an ionization energy of 70 eV was employed for detection of volatile derivatives. Helium was used as the carrier gas at constant flow rate 1 mL/min, with linear velocity of 29.6 cm/s and split ratio was 1:20. Temperature program for the column included: the initial oven temperature was kept at 50 °C for 3 min, then temperature was raised to 265 °C with a program ramp rate of 2.5 °C/min. It was maintained at ultimate temperature for 6 min. The temperature of the injector was 250 °C. Identification of fatty acids was performed by comparing FAME peaks relative mass spectra with those obtained from the Wiley 229, Nist 107, Nist 21, and Adams 2007 Libraries. Results were expressed as Mass response area in relative percentages.15
Free radical scavenging assay
DPPH with molecular formula of C18H12N5O6 was used to evaluate the in vitro antioxidant activity of O. acanthium methanol extract and fractions, with quercetin serving as the reference positive control. Initially, various concentrations of the samples were prepared and eight milligrams of DPPH was dissolved in methanol to achieve a concentration of 80 µg/mL. Each of the diluted sample solutions (1 mL), were mixed with DPPH (1 mL) and were allowed to stand for 30 min for any oxidation reaction to occur. Finally, UV absorbance of the samples were recorded at 517 nm using a spectrophotometer, as per previous protocols.16 The experiments were performed in triplicate and the average absorption was noted for each concentration. Ultimately, the percentage of DPPH radical scavenging activity is calculated together with the IC50 value, the concentration of a sample required to scavenge 50% of the DPPH radicals which stands for the half maximal inhibitory concentration of each sample, which is calculated and reported from the slope of concentration-inhibition percentage curve constructed. Likewise, the same procedure was followed for the positive control, quercetin. Results of this assay were indicative of the antioxidant potential of the O. acanthium extract and fractions compared to the reference compound quercetin.
Results and Discussion
Structure elucidation
Compounds from 40% SPE fraction of methanol extract
Compound I (31 mg) occurred as dark brownish crystals with retention time of 10.9 minutes in HPLC chromatogram. The 1HNMR spectrum of the compound exhibited some clear peaks at 7.62 ppm (d, 15.47 Hz), 7.57 ppm (d, 15.49 Hz), 6.78 ppm (d, 8.2 Hz), 6.39 ppm (d, 15.47 Hz), 6.30 ppm (d, 15.49 Hz), and 7.38 ppm (d, 3.18 Hz). Moreover, a signal at 5.38 ppm with coupling constant of 3.4 Hz revealed that there might be an aldose moiety in the structure. 13CNMR spectrum confirmed presence of a lignan lactone ring (36, 46, 71, and 180 ppm). Also, the presence of 2 caffeoyl groups was revealed via observation of characteristic peaks at 167.5-168.0, 126.4-126.7, 114.6-115.0, and 147.9-148.0 ppm. No methoxy groups were present at this compound. Maximum UV absorbance was recorded at 245, 295, and 327 nm which are in agreement with the caffeoyl groups’ absorbance pattern. Finally, compound 1 was suggested to be 2’’,6’’-[dicaffeoyl]-glucosyl-4’-hydroxy enterolactone (Figure 1).
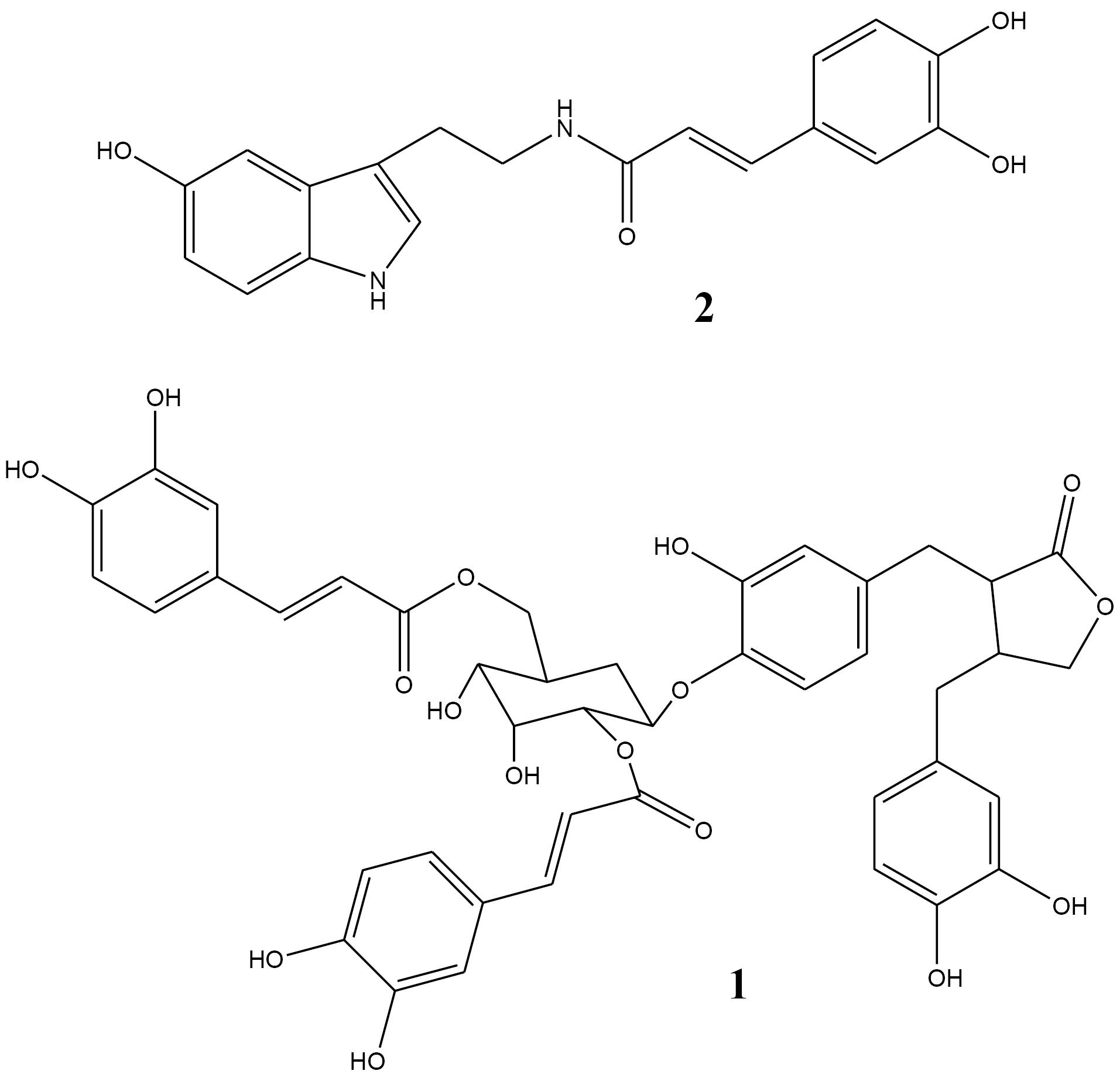
Figure 1.
Isolated compounds from 40% SPE fraction of Onopordum acanthium seeds methanol extract
.
Isolated compounds from 40% SPE fraction of Onopordum acanthium seeds methanol extract
Compound II (3 mg) was obtained as light brown crystals with retention time of 52 minutes in HPLC chromatogram. 1HNMR spectrum showed main signals at 7.39 ppm (t, 15.6 Hz), 7.0 ppm (s), 6,97 ppm (dd, 1.9 and 7.67 Hz), 6.89 ppm (d, 1.9 Hz), 6.76 ppm (d, 8.64 Hz), 6.64 ppm (dd, 1.95 and 8.13 Hz), 6.34 ppm (d, 15.68 Hz), 3.57 ppm (t, 7.07 Hz), 2.93 ppm (t, 7.07 Hz). These data showed that caffeoyl and serotonin moieties are present in the skeleton. Also, the presence of aromatic, olefinic, and ketone signals in 13CNMR spectrum and UV absorbance together with data reported in the literature, confirmed that the molecule could be N-caffeoyl serotonin (Figure 1).
Compounds from 60% SPE fraction of methanol extract
Preparative HPLC chromatogram of 60% SPE fraction of methanol extract indicated 15 major peaks, which 5 of them were purified and their structure were elucidated.
Compound III (142 mg) was purified as a crystalline compound with retention time of 5.5 minutes. This compound showed similar signals and absorbance to compound 2 and the presence of acyl and serotonin moieties was confirmed. But a singlet signal was appeared at 6.99 ppm at 1HNMR spectrum. It means that there are 3 OH- substitutes on the acyl side on the aromatic ring. So, the structure was elucidated as 3’,4’,5’-trihydroxy-cynnamoyl-serotonin (Figure 1).
Compound IV (47 mg) showed two groups of proton signals at 2.5-4.5 and 6.5-7.5 ppm. In the aromatic region, three signals belonging to a benzene ring are observed. Another three aromatic signals are clear in this region. Observed signals at 3.5-4.5 ppm are related to an aldose moiety. Also, 3 methyl groups appeared in 3.74, 3.76, and 3.78 ppm as singlets indicating methoxy substitutes. Signals from 13CNMR spectrum confirm the presence of mentioned aromatic rings, aldose, and methoxy groups. Comparison of obtained data with those of the literature17 indicated that compound IV was a lignan named arctiin (Figure 2). Arctiin is a lignan that is very common in Asteraceae family and has been isolated from Onopordum spp., and also from fruits, leaves and roots of Centaurea americana and Centaurea imperialis. Arctiin has also been isolated from Trachelospermum asiaticum, Saussurea heteromalla, and Forsythia viridissima. It is reported from O. acanthium for the first time. In previous studies, arctiin and its genin have shown anti-inflammatory, anti-mutagenic, cytotoxic, and antitumor activities.18-20 Elsewhere, arctiin revealed to be an inhibitor of PC-3 human prostate cancer cells, however its mechanism of action remained unknown.
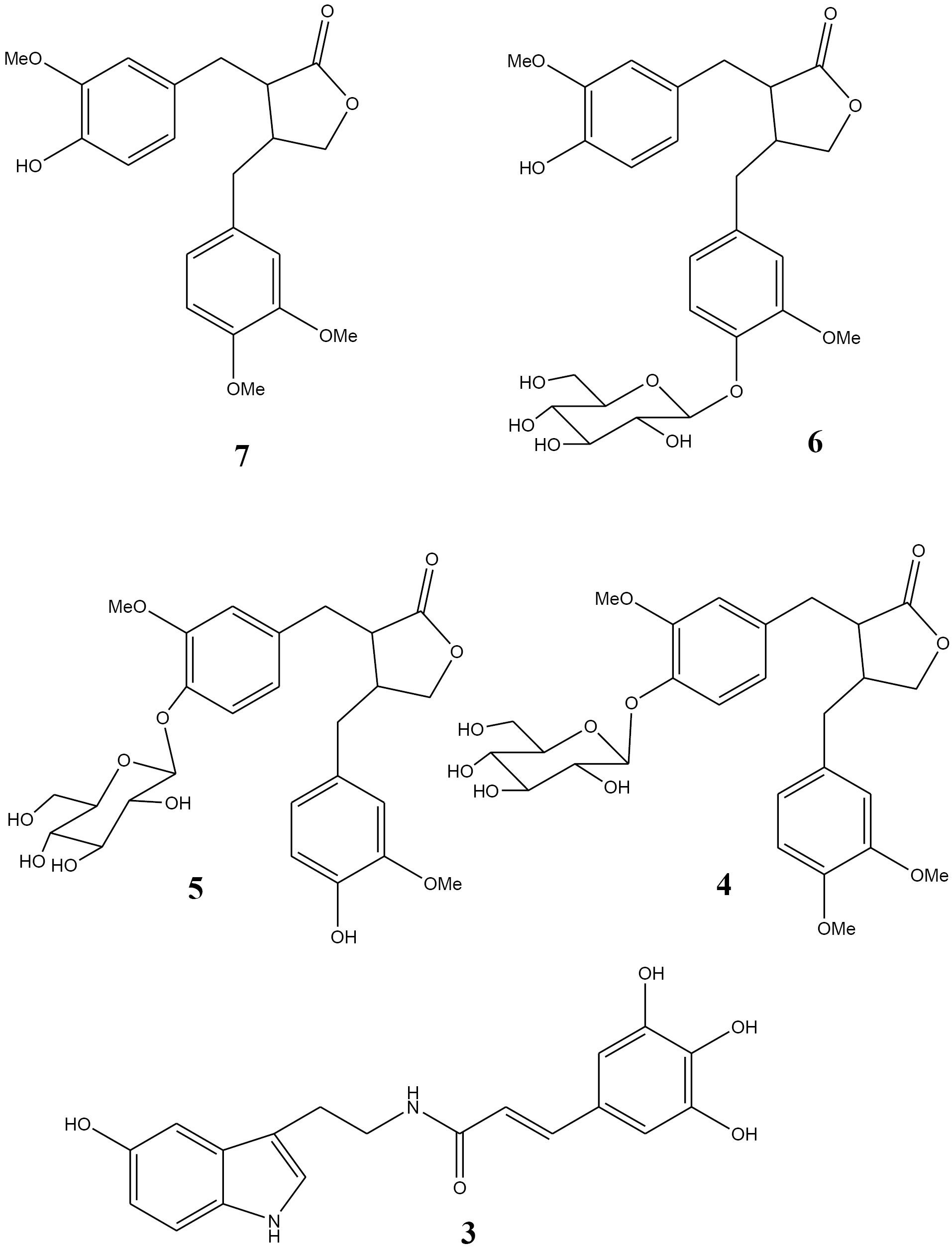
Figure 2.
Isolated compounds from 40% SPE fraction of Onopordum acanthium seeds methanol extract
.
Isolated compounds from 40% SPE fraction of Onopordum acanthium seeds methanol extract
Compound V (182 mg) showed very similar NMR spectra to compound IV. In 1HNMR spectrum the methyl signal at 3.74 ppm was disappeared. Also, in 13CNMR spectrum just two methoxy groups were found (55.1 1nd 55.3 ppm). This compound was identified as matairesinoside (Figure 2). It was previously isolated from the genus Centaurea. This is the first report on the isolation of matairesinoside from Onopordum spp. Cytotoxicity and anti-inflammatory activity have been reported for this lignan metabolite in previous studies.21
Compound VI (15 mg) with retention time of 12 min exhibited proton signals very close to those of arctiin. The only difference was the shift of a doublet peak to 7 ppm which showed the attachment of glucose to C4’. So, it was characterized as matairesinol-4’-glucoside (Figure 2). Matairesinol (Matairesinol-4’-glucoside) has been isolated from different species of Asteraceae, Apiaceae, Oleaceae, and other families. To the best of our knowledge, this is the first report on the purification of matairesinol from Onopordum members.
Compound VII (6 mg) with retention time of 27 min showed that it contains 2 aromatic rings with the same substitution pattern. Existence of 3 methoxy groups was confirmed by 3 singlet signals at 3.75, 3.77, and 3.80 ppm. Moreover, multiples around 3.9-4.3 ppm and 2.5-3 ppm affirmed the presence of lignans lactone ring. Also, 13CNMR data supported the lignan structure with 2 aromatic rings and their substituted 3 methoxy groups. Finally, this compound was identified as arctigenin (Figure 2). It was reported from O. acanthium and O. acaulon before. Arctigenin has great biological properties including anti-inflammatory, antiviral, and anticancer activities.22-25
The isolated lignan derivatives include arctiin, matairesinoside, matairesinole-4’-glucoside, arctigenin, and 2’’,6’’-[dicaffeoyl]-glucosyl-4’-hydroxy-enterolactone (Figures 1 and 2). These lignan derivatives belong to phenylpropanoid dibenzylbutyrolactone lignans (with two β-β’ bonds in C3 and C6 and a lactone bridge of γ-γ’). Matairesinol glycosylation gives matairesinoside, the methylation of which leads to the formation of arctiin. Arctiin is converted to arctigenin by a β-glucosidase. Lignans possess antifungal, antibacterial, antitumor, anti-inflammatory, and pesticide activities. Also, from ecologic point of views, lignans are evolutionary biomarkers and have critical roles in the interactions of plants with other organisms.
Identification of fatty acid profile
Lipids are necessary in the human diet for the body due to their vital role in providing energy and essential fatty acids for the body’s health.
The oil of O. acanthium from Iranian origin was obtained for the first time by soxhlet extraction using petroleum ether and its fatty acid composition was investigated. Fatty acid composition was determined using GC/MS analysis after methylation of related acid (Table 1). The total content of thistle lipid was 30.55% w/w. eight fatty acids were identified that make up 100% of the oil composition. The main component was identified as 9,12-Octadecadienoic acid (63.41%) followed by 13-Octadecenoic acid (28.21%) and n-hexadecanoic acid (5.86%). Chemical structures of the identified fatty acids could be seen in Figure 3. The ratio of unsaturated/saturated FA was 91.62: 8.38. The oil yield in this work is higher than a previous work on Bulgarian thistle (21.2% w/w) but the unsaturated/saturated is similar (88.5:11.5).26 There are few reports on the fatty acid and lipid composition of O. acanthium seeds, but other Onopordum species have been neglected. Turkish O. acanthium was investigated for fatty acid, tocopherol, and mineral contents of its seeds. Similar percentages of palmitic acid (5.8) and linoleic acid (65.9) was reported in that work. But at the present study, 13-octadecenoic acid was detected instead of oleic acid (9-octadecenoic acid) which was the second major compound in Turkish thistle (18.8%).
Table 1.
Fatty acid composition of petroleum ether extract of Onopordum acanthium
|
Fatty acid
|
|
Percentage
|
Molecular weight
|
Formula
|
| 3-Methyltetradecanoic acid |
C15:0 |
0.08 |
242.403 g/mol |
C15H30O2 |
| Palmitic acid |
C16:0 |
5.86 |
256.430 g/mol |
C16H32O2 |
| Linoleic acid |
C18:2 |
63.41 |
280.452 g/mol |
C18H32O2 |
| 13-Octadecenoic acid |
C18:1 |
28.21 |
282.468 g/mol |
C18H34O2 |
| Stearic acid (Octadecanoic acid) |
C18:0 |
3.73 |
284.484 g/mol |
C18H36O2 |
| Methyl-9,10-epoxystearic acid |
C18:0 |
0.2 |
298.490 g/mol |
C18H34O3 |
| Arachidic acid (Eicosanoic acid) |
C20:0 |
0.42 |
312.538 g/mol |
C20H40O2 |
| Lignoceric acid (Tetracosanoic acid) |
C24:0 |
0.1 |
368.646 g/mol |
C24H48O2 |
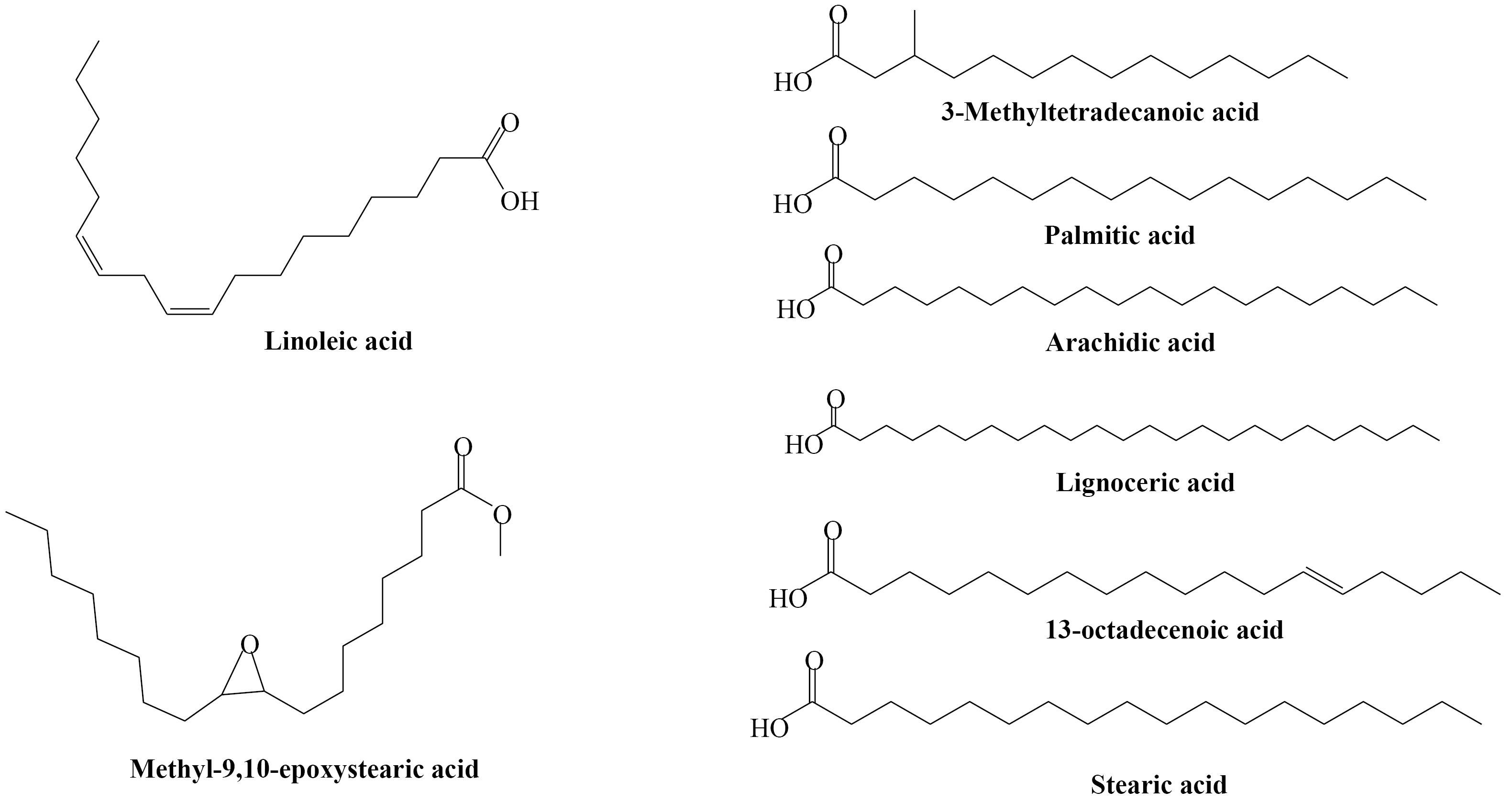
Figure 3.
Fatty acids identified in the seed oil of Onopordum acanthium
.
Fatty acids identified in the seed oil of Onopordum acanthium
Radical scavenging activity
The results of the DPPH assay revealed that all methanol fractions of O. acanthium exhibited strong radical scavenging activities, with RC50 values ranging from 2.1 to 501 µg/mL. In particular, the 80% methanol fraction demonstrated the most prominent antioxidant activity, as is shown in Table 2. The antioxidant capacity of the tested samples was found to be comparable to that of quercetin. This notable antioxidant potential might be attributed to the high levels of lignans and serotonin derivatives present in the methanol extract. Both lignans and serotonin derivatives belong to the classes of compounds that contain phenolic groups, which are well-known in playing vital antioxidant roles through serving as electron and hydrogen donors, metal chelators, and reducing agents. In the same way, the observed antioxidant effects might also be attributed to the presence of other secondary metabolites such as flavonoids and organic acids, that are also acknowledged to be present in thistles. These findings suggest that thistles possess significant antioxidant properties due to the presence of various bioactive compounds that contribute to their radical scavenging activities.
Table 2.
Antioxidant activity of fractions obtained from methanolic extract of Onopordum acanthium (RC50 µg/mL)
|
Sample
|
|
Fr-40%
|
Fr-60%
|
Fr-80%
|
Fr-100%
|
Quercetin
|
| 8.3 ± 0.14 |
9.2 ± 0.15 |
2.6 ± 0.08 |
501.9 ± 20.1 |
2.3 ± 0.06 |
Conclusion
In conclusion, the present study highlights the potential health benefits of Scotch thistle (O. acanthium L.) due to its high content of lignans including arctiin, arctigenin, and matairesinol, serotonin derivatives, and antioxidant activity. Additionally, the fatty acid profile of Scotch thistle provides valuable information for its potential use in functional foods and nutraceuticals. The findings of this research provide valuable insight into the bioactive compounds present in O. acanthium, a plant traditionally utilized in Iran, Azerbaijan, and Turkey for the treatment of infectious and hemorrhaging hemorrhoids. Nonetheless, further research is warranted to fully understand the mechanisms underlying these beneficial effects and to explore the potential applications of Scotch thistle in the development of novel therapeutic agents. Overall, Scotch thistle emerges as a promising natural resource with a wide range of health-promoting properties that warrant further investigation and utilization.
Competing Interests
The authors declare that they have no competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The partial financial support of this study by Tabriz University of Medical Sciences is gratefully acknowledged.
References
- Bruno M, Maggio A, Rosselli S, Safder M, Bancheva S. The metabolites of the genus Onopordum (Asteraceae): chemistry and biological properties. Curr Org Chem 2011; 15(6):888-927. doi: 10.2174/138527211794518880 [Crossref] [ Google Scholar]
- Robertovna GE, Alexeevich KD, Alexeevich SA, Petrovna GM, Kenzhebaevna OK. A traditional medicine plant, Onopordumacanthium L (Asteraceae): chemical composition and pharmacological research. Plants (Basel) 2019; 8(2):40. doi: 10.3390/plants8020040 [Crossref] [ Google Scholar]
- Al-Snafi AE. Constituents and pharmacology of Onopordumacanthium. IOSR J Pharm 2020; 10(3):7-14. [ Google Scholar]
- Wei C, Zhou S, Shi K, Zhang C, Shao H. Chemical profile and phytotoxic action of Onopordumacanthium essential oil. Sci Rep 2020; 10(1):13568. doi: 10.1038/s41598-020-70463-7 [Crossref] [ Google Scholar]
- Zare K, Nazemyeh H, Lotfipour F, Farabi S, Ghiamirad M, Barzegari A. Antibacterial activity and total phenolic content of the Onopordonacanthium L seeds. Pharm Sci 2014; 20(1):6-11. [ Google Scholar]
- Shahcheraghi SN, Sadat Shandiz SA, Pakpour B. An eco-friendly fabrication of silver chloride nanoparticles (AgClNPs) using Onopordumacanthium L extract induces apoptosis in breast cancer MDA-MB-232 cells. Bionanoscience 2022; 12(2):339-50. doi: 10.1007/s12668-022-00970-6 [Crossref] [ Google Scholar]
- Hassanzadeh M, Sharifi N, Mahernia S, Rahimi N, Dehpour AR, Amanlou M. Effects of onopordia, a novel isolated compound from Onopordonacanthium, on pentylenetetrazole-induced seizures in mice: possible involvement of nitric oxide pathway. J Tradit Complement Med 2021; 11(1):22-6. doi: 10.1016/j.jtcme.2019.11.005 [Crossref] [ Google Scholar]
- Arfaoui MO, Renaud J, Ghazghazi H, Boukhchina S, Mayer P. Variation in oil content, fatty acid and phytosterols profile of Onopordumacanthium L during seed development. Nat Prod Res 2014; 28(24):2293-300. doi: 10.1080/14786419.2014.940944 [Crossref] [ Google Scholar]
- dos Santos Maia M, Raimundo e Silva JP, de Lima Nunes TA, Saraiva de Sousa JM, Rodrigues GC, Messias Monteiro AF. Virtual screening and the in vitro assessment of the antileishmanial activity of lignans. Molecules 2020; 25(10):2281. doi: 10.3390/molecules25102281 [Crossref] [ Google Scholar]
- Draganescu D, Andritoiu C, Hritcu D, Dodi G, Popa MI. Flaxseed lignans and polyphenols enhanced activity in streptozotocin-induced diabetic rats. Biology (Basel) 2021; 10(1):43. doi: 10.3390/biology10010043 [Crossref] [ Google Scholar]
- Nurbek S, Murata T, Suganuma K, Ishikawa Y, Buyankhishig B, Kikuchi T. Isolation and evaluation of trypanocidal activity of sesquiterpenoids, flavonoids, and lignans in Artemisia sieversiana collected in Mongolia. J Nat Med 2020; 74(4):750-7. doi: 10.1007/s11418-020-01429-2 [Crossref] [ Google Scholar]
- Mottaghi S, Abbaszadeh H. A comprehensive mechanistic insight into the dietary and estrogenic lignans, arctigenin and sesamin as potential anticarcinogenic and anticancer agents Current status, challenges, and future perspectives. Crit Rev Food Sci Nutr 2022; 62(26):7301-18. doi: 10.1080/10408398.2021.1913568 [Crossref] [ Google Scholar]
- Baştürk A, Peker S. Antioxidant capacity, fatty acid profile and volatile components of the Onopordumanatolicum and Onopordumheteracanthum species seeds grown in Van, Turkey. J Inst Sci Technol 2021; 11(4):2810-22. doi: 10.21597/jist.895713 [Crossref] [ Google Scholar]
- Dimitrov D, Parzhanova A, Ivanova S. Study of the volatile fraction of distillates with added donkey thistle (Onopordumacanthium L) extracts. J Cent Eur Agric 2021; 22(1):96-103. doi: 10.5513/jcea01/22.1.2703 [Crossref] [ Google Scholar]
- Bahadori MB, Maggi F, Zengin G, Asghari B, Eskandani M. Essential oils of hedgenettles (Stachys inflata, S lavandulifolia, and S byzantina) have antioxidant, anti-Alzheimer, antidiabetic, and anti-obesity potential: a comparative study. Ind Crops Prod 2020; 145:112089. doi: 10.1016/j.indcrop.2020.112089 [Crossref] [ Google Scholar]
- Bahadori MB, Zengin G, Dinparast L, Eskandani M. The health benefits of three Hedgenettle herbal teas (Stachys byzantina, Stachys inflata, and Stachys lavandulifolia) - profiling phenolic and antioxidant activities. Eur J Integr Med 2020; 36:101134. doi: 10.1016/j.eujim.2020.101134 [Crossref] [ Google Scholar]
- Zhou B, Wang L, Liang Y, Li J, Pan X. Arctiin suppresses H9N2 avian influenza virus-mediated inflammation via activation of Nrf2/HO-1 signaling. BMC Complement Med Ther 2021; 21(1):289. doi: 10.1186/s12906-021-03462-4 [Crossref] [ Google Scholar]
- Xu X, Zeng XY, Cui YX, Li YB, Cheng JH, Zhao XD. Antidepressive effect of arctiin by attenuating neuroinflammation via HMGB1/TLR4- and TNF-α/TNFR1-mediated NF-κB activation. ACS Chem Neurosci 2020; 11(15):2214-30. doi: 10.1021/acschemneuro.0c00120 [Crossref] [ Google Scholar]
- Zhou Y, Lu X, Xia L, Yao W, Qin G, Wang G. [Arctiin antagonizes triptolide-induced renal toxicity in rats via anti-inflammatory pathway]. Nan Fang Yi Ke Da Xue Xue Bao 2020; 40(10):1399-405. doi: 10.12122/j.issn.1673-4254.2020.10.04 [Crossref] [ Google Scholar]
- Ge L, Liu F, Hu Y, Zhou X. Qualitative and quantitative analysis of arctiin and arctigenin in Arctium tomentosum Mill by high-performance thin-layer chromatography. J Planar Chromatogr Mod TLC 2020; 33(1):19-26. doi: 10.1007/s00764-019-00005-z [Crossref] [ Google Scholar]
- Demiroz T, Nalbantsoy A, Kose FA, Baykan S. Phytochemical composition and antioxidant, cytotoxic and anti-inflammatory properties of Psephellusgoeksunensis (Aytaç & H Duman) Greuter & Raab-Straube. S Afr J Bot 2020; 130:1-7. doi: 10.1016/j.sajb.2019.11.021 [Crossref] [ Google Scholar]
- Batool A, Miana GA, Alam M, Khan MT, Muddassir M, Zaman W. Bioassay-guided fractionation and isolation of arctigenin from Saussureaheteromalla for in vitro and in silico cytotoxic activity against HeLa cells. Physiol Mol Plant Pathol 2022; 117:101749. doi: 10.1016/j.pmpp.2021.101749 [Crossref] [ Google Scholar]
- Wu L, Chen J, Zhou D, Chen R, Chen X, Shao Z. Anti-inflammatory activity of arctigenin against PCV2 infection in a mouse model. Vet Med Sci 2022; 8(2):700-9. doi: 10.1002/vms3.693 [Crossref] [ Google Scholar]
- Shen YF, Liu YH, Li BY, Liu TQ, Wang GX. Evaluation on antiviral activity of a novel arctigenin derivative against multiple rhabdoviruses in aquaculture. Virus Res 2020; 285:198019. doi: 10.1016/j.virusres.2020.198019 [Crossref] [ Google Scholar]
- Okubo S, Ohta T, Shoyama Y, Uto T. Arctigenin suppresses cell proliferation via autophagy inhibition in hepatocellular carcinoma cells. J Nat Med 2020; 74(3):525-32. doi: 10.1007/s11418-020-01396-8 [Crossref] [ Google Scholar]
- Slavov I, Merdzhanov P, Angelova-Romova M, Zlatanov M, Antova G, Dimitrova-Dyulgerova I. Lipid composition of Carduus thoermeri Weinm, Onopordumacanthium L and Silybum marianum L, growing in Bulgaria. Bulg J Agric Sci 2014; 20(3):622-7. [ Google Scholar]