Pharmaceutical Sciences. 2025;31(3):304-312.
doi: 10.34172/PS.025.40919
Research Article
Bioactivity-guided Isolation of Anticancer Constituents of Strophanthus hispidus DC. (Apocynaceae) Whole plant
Owoola A. Ambali Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing, 1, 2, 3 
Emmanuel O. Yeye Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing, 2 
Edith O. Ajaiyeoba Conceptualization, Methodology, Project administration, Resources, Supervision, 1 
Omonike O. Ogbole Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – review & editing, 1, * 
Iqbal. M. Choudhary Formal analysis, Methodology, Project administration, Resources, Supervision, Validation, Visualization, 2 
Author information:
1Department of Pharmacognosy, Faculty of Pharmacy, University of Ibadan, Ibadan, Nigeria
2International Centre for Chemical and Biological Sciences, University of Karachi, Karachi 75270, Pakistan
3Department of Biomedical Sciences, School of Medicine and Allied Health Sciences, University of the Gambia, Banjul, The Gambia
Abstract
Background:
Strophanthus hispidus DC; a medicinal plant in the Apocynaceae family was reported to be effective in the management of cancer through an ethnobotanical survey conducted in Ibadan, Nigeria. This study explicitly explored the cytotoxic potential of the whole plant of Strophanthus hispidus leading to the isolation of cytotoxic secondary metabolites.
Methods:
The crude extract of S. hispidus was prepared using the Soxhlet apparatus and concentrated using the rotary evaporator. The crude extract obtained from S. hispidus was then partitioned into n-Hexane, dichloromethane (DCM), ethyl acetate and butanol fractions for further study. Compounds were isolated by column chromatography, normal and reverse-phase high performance liquid chromatography, and structures were determined by spectroscopic methods. The crude extracts, fractions and isolated compounds were screened for cytotoxicity against breast (AU565, MCF-7), cervical (HeLa), and skeletal muscle (RD) with two normal cell lines: rat and human fibroblast (BJ and 3T3) for selectivity using the MTT assay. Doxorubicin and cycloheximide served as standards for the assay.
Results:
The methanol extract and DCM fraction showed significant cytotoxicity with IC50 values (Mean±SEM) of 1.86±0.01 µg/mL, 0.32±0.01 µg/mL and 1.26±0.06 µg/mL, 0.21±0.06 µg/mL against AU565 and HeLa respectively. The bioassay-guided isolation of cytotoxic compounds from S. hispidus afforded two new compounds (1, 2) alongside six other known compounds (3-8). The compounds were identified as 16-hydroxy-17-(1-hydroxyethyl)-13,17-dimethyl-5H-cyclopenta[a]phenanthrene-4,6-dione (1), 2-(4-ethoxy-3-methoxyphenyl)-5-hydroxy-3,7,8-trimethoxy-4H-chromen-4-one (2); Stigmasterol (3); β-Sitosterol-β-D-glucoside (4); pityriacitrin (5); ursolic acid (6); helveticoside (7) and Urs-12-en-28-oic acid,2,3,23-trihydroxy-(2β,3β,4α) (8). Compounds 1, 4, 6, 7 and 8 displayed significant cytotoxicity and selectivity towards cancer and normal cell lines, respectively.
Conclusion:
This study further confirms that S. hispidus is cytotoxic with the DCM fraction being the most significantly cytotoxic and selective to normal cells. This is the first report on some characterized compounds from S. hispidus. In addition, these secondary metabolites can serve as leads in cancer drug development.
Keywords: Strophanthus hispidus, Apocynaceae, Cancer cell lines, Ursolic acid, Helveticoside
Copyright and License Information
© 2025 The Author(s).
This is an open access article and applies the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This research was funded by a TWAS fellowship given to Owoola Azeezat AMBALI (Award number fr 3240299160).
Introduction
With the increasing demands for drugs for the treatment of cancer in low- and middle-income countries, new areas are provided for the utilization of medicinal plants and their products. Researches have shown that Nigeria is a country with a wide flora of medicinal plants with therapeutic values.1,2 Cancer is ranked as the second leading cause of death3-5 with several causes including environmental,6,7 heredity8,9 and lifestyle.10 In Nigeria, the prevalent types of cancer include cervical, breast, and prostate.11
Natural products are known to possess a great range of bioactive constituents in the form of secondary metabolites for disease management.12,13 Studies have shown useful compounds such as vincristine and vinblastine from Catharanthus roseus as chemotherapeutic means of cancer treatment.14,15 Several means of treatment using natural products from plants, animals and sometimes microorganisms have been evaluated.16,17 An in-depth assessment of these could provide a clue to effective drugs for the treatment of diseases such as cancer.
Strophanthus hispidus DC belongs to the family of Apocynaceae with the common name Brown Strophanthus and the local name Sagere. This was obtained from a previous ethno-botanical survey conducted for plants used in the traditional treatment of cancer.18 The plant, a climbing shrub which sometimes appears as a liana, is found in Western African countries including Nigeria, Ghana, and sometimes in other African countries such as Congo, Uganda and Cabinda.19-21 Traditionally, the juice from the seeds are used as an arrow poison, also used to treat snake bites and skin infections.22 The potency of the plant in the treatment of cardiovascular diseases23 could be a result of the presence of cardenolide including K-strophanthoside, K-strophanthin-β from S. kombe. Ursolic acid, a triterpene previously isolated from S. specious has been reported for great anticancer activity.24 Boivinide A isolated from the ethanol extract of S. boivinii showed significant anti-proliferative activity against the human ovarian cancer cell line.25
The present study describes the isolation and identification of potent cytotoxic constituents from S. hispidus DC, and their cytotoxic properties against selected cancer cell lines using the MTT assay.
Methods
Plant collection and identification
Fresh whole plant of S. hispidus was obtained from Ipara-Remo in Ogun state, Nigeria between June and September. The plant material was identified and authenticated at the Forest Herbarium Ibadan, Nigeria, with a voucher number- FHI-112443 assigned. The plant specimen was then deposited in its Herbarium. According to the International Union of Conservation of Nature, S. hispidus was categorized as least concerned.
Extraction and isolation
The air-dried and powdered plant material of S. hispidus (200 g) was extracted using a Soxhlet apparatus with MeOH in 4 cycles at 65°C using 500 mL of MeOH at each cycle. The liquid extract (continuous hot extract) obtained was thereafter concentrated at a reduced pressure and temperature of 40 °C using the BUCHI rotary evaporator (R-100 rotavapor) which gave a greenish-brown paste. Approximately 2 Kg of S. hispidus yielded 135.4 g of crude extract. A 100 g of crude extract was reconstituted in 200 mL MeOH/ Water (3:1); thereafter, successive liquid-liquid extraction was carried out using n-hexane (300 mL x 4), dichloromethane (DCM) (300 mL x 4), ethyl acetate (EtOAc) and butanol (300 mL x 4) using a separating funnel.
The n-hexane fraction of crude extract of S. hispidus was purified using the column chromatographic method. Compounds-3 SHH-14 (5 mg, Rf 0.5) and -4 SHH-32 (10 mg, Rf 0.6) were obtained as pure isolates directly by the adsorption column chromatographic method (5 x 50 cm) using solvent systems n-hexane: ethyl acetate (80:20) and ethyl acetate: methanol (90:10) respectively. Fraction SHH-19 obtained from the preliminary column was loaded for further purification on column (5 × 30 cm) packed with 20 g of silica gel to obtain sub-faction and a final purification on Japan Analytical Industry Co. Ltd preparative normal phase HPLC (LC-908W, SIL-60A) using n-hexane: EtOAc 70: 30 with 1% acetic acid to obtain compound-2, SHH-42-4 (1.5 mg, TR of 62 min, Rf 0.5). The DCM fraction was purified using the adsorption column chromatographic method with elution carried out with solvent systems of n-hexane: DCM and then n-hexane: ethyl acetate in increasing polarity. A total of 126 fractions were obtained and pooled by TLC analysis into 25 major fractions. Further purifications were carried out using smaller column (2.5 × 30cm) and or high-performance liquid chromatography (HPLC) respectively. Compound-5 ShD-58 (20 mg, Rf 0.58), Compound-6, ShD-9 (50 mg, Rf 0.5), Compound-7, ShD-21 (7 mg, Rf 0.5), were isolated using solvent systems n-hexane: DCM 70:30, n-hexane: EtOAc 70:30; EtOAc: Methanol 90:10 with column size 5 × 50 cm respectively. Compound-8 ShD-26-MF3, was isolated by Japan Analytical Industry Co. Ltd preparative normal phase HPLC (LC-908W, SIL-60A) with DCM: MeOH 95:5 (TR of 15 min, Rf: 0.8) while Compound-1, ShD-MF-23B was isolated on Japan Analytical Industry Co., Ltd semi-preparative reverse phase HPLC (ODS-H-80, MeOH: H2O 95:5 (TR of 15 min, Rf: 0.54). The TLC plates were observed under UV light after development and then sprayed with sulphuric acid or Dragendorff’s reagent to resolve the classes of compounds.
General experimental procedure
NMR data was recorded on Bruker Avance III spectrometer operating at 500 and 400 MHz (1H) and 100 MHz (13C) using standard pulse sequences referenced to residual solvent signals. The HRMS were measured using LTQ Orbitrap spectrometer (Thermo Scientific, USA) equipped with a HESI-II source. The ultraviolet (UV) data were recorded and analysed on THERMO ELECTRON-VISION pro software V4.10 in MeOH. The infrared, IR spectrum was recorded using KBr pellets on BRUKER VECTOR 22 (FT-IR-1). Melting points were recorded on BUCHI M-560. Analytical TLC was carried out on a pre-coated silica gel aluminium plate 60 F254 visualised at 254 nm (Merck). A Multiskan microplate spectrophotometer (Thermo Scientific) was used for the absorbance measurement for the MTT assay.
Cell lines and cytotoxicity assay
The cancer cell lines purchased from American Type Culture Collection (ATCC), were seeded in 96-well plates at the density of 6 × 104 cells/mL followed by incubation at 37 °C overnight in a humidified CO2 incubator. Cell culture material included Dulbecco’s Modified Eagle Medium (DMEM), 10% fetal bovine serum (FBS) and trypsin were obtained from Biosera (Nouaille, France). The plant extract, fractions and compounds were dissolved in DMSO and then added to the monolayer of cells at ten-fold concentration range of 1000 - 0.01 µg/mL followed by incubation at 37 °C for 24 h. Doxorubicin and cycloheximide were used as positive control while negative control contained the growth media and DMSO. After 48 h, 200 µL of MTT (0.5 mg/mL) was added to each well and then incubated for 4 h. Thereafter, 100 µL of DMSO was added to each well and then measured at an absorbance of 570 nm, using a microplate reader (Spectra Max Plus, Molecular Devices, CA, USA). Each experiment was carried out in triplicate and cell viability was calculated as
where A represents the mean optical density of untreated cells while B is of treated cells respectively.26
Data presentation and statistical analysis
Cytotoxicity was recorded as concentration causing 50% growth inhibition (IC50) for all cell lines with Microsoft Excel version 2016 using data from the three independent experiments. The results obtained were expressed as mean ± standard error of the mean (SEM). Statistical analysis was carried out using GraphPad Version 5.01. (GraphPad Prism Software Inc., San Diego, CA). Data obtained were subjected to one-way analysis of variance (ANOVA) and then Tukey’s test for statistical analysis with a P value of less than 0.05 considered statistically significant. The selective index was calculated as a ratio of IC50 value of the normal cell to those of cancer cell lines.
Results and Discussion
Breast and cervical cancers have been most commonly diagnosed in sub-Saharan African women over the last decade,27 thus the choice of adenocarcinomas used for the study.
To explore the biological activity of S. hispidus with human cancer cells, we first extracted the whole plant with methanol, and then examined its cytotoxicity in terms of cell viability in four different cancer cells; AU565, MCF-7, HeLa, RD, and two normal cells; BJ and 3T3 for selectivity. After 72 hours of treatment, the MTT assay revealed that the crude extract drastically reduced the cell viability of the cancer cells in a dose-dependent manner with a range of IC50 0.32 to 1.86 µg/mL as shown in Table 1. The observations further confirmed the usage of S. hispidus in the management of cancer by traditional medical practitioners.
Table 1.
The in vitro cytotoxic activity of S. hispidus crude extract and fractions
|
Sample
|
AU565
|
HeLa
|
BJ
|
|
IC50
±SEM (µg/mL)
|
IC50
±SEM (µg/mL)
|
IC50
±SEM (µg/mL)
|
| ShCrude |
1.86 ± 0.01# |
0.32 ± 0.01# |
4.19 ± 0.10# |
| ShH |
10.72 ± 0.18 |
11.91 ± 1.25 |
14.26 ± 0.20 |
| ShD |
1.26 ± 0.06# |
0.21 ± 0.06# |
2.36 ± 1.10 |
| ShE |
8.13 ± 0.22 |
1.6 ± 0.02 |
4.23 ± 1.10 |
| ShAq |
18.55 ± 2.50 |
1.7 ± 0.5 |
3.1 ± 1.10 |
| Doxorubicin* |
0.54 ± 0.04 |
1.2 ± 0.01 |
2.84 ± 0.02 |
ShH: Strophanthus hispidus Hexane; ShD: Strophanthus hispidus DCM; ShE: Strophanthus hispidus Ethyl acetate fraction; ShAq: Aqueous fraction of Strophanthus hispidus. HeLa: Cervical carcinoma AU565: Breast adenocarcinoma. *Standard drug for the study; # The mean difference is significant at P < 0.05 with respect to standard drug.
For further investigations on the cytotoxicity of S. hispidus, the crude extract was partitioned into four different fractions based on the polarity of the solvents. To make a distinction for the fraction that exerted cytotoxicity, each fraction was tested against cell lines AU565 and HeLa, the n-hexane and DCM fractions showed significant cytotoxicity with IC50 values (Table 1) for both cell lines. These fractions were then subjected to further investigations using repeated column chromatography and HPLC purification leading to the isolation of eight compounds. The compounds were identified as 16-hydroxy-17-(1-hydroxyethyl)-13,17-dimethyl-5H-cyclopenta[a]phenanthrene-4,6-dione (1); 2-(4-ethoxy-3-methoxyphenyl)-5-hydroxy-3,7,8-trimethoxy-4H-chromen-4-one (2); stigmasterol (3)28; β-sitosterol-β-D-glucoside (Daucosterol) (4)29; pityriacitrin (5)30; ursolic acid (6)24; strophanthidin- digitoxoside (Helveticoside) (7)31; and Urs-12-en-28-oic acid, 2,3,23-trihydroxy-(2β,3β,4α) (8)32 as shown in Figure 1.
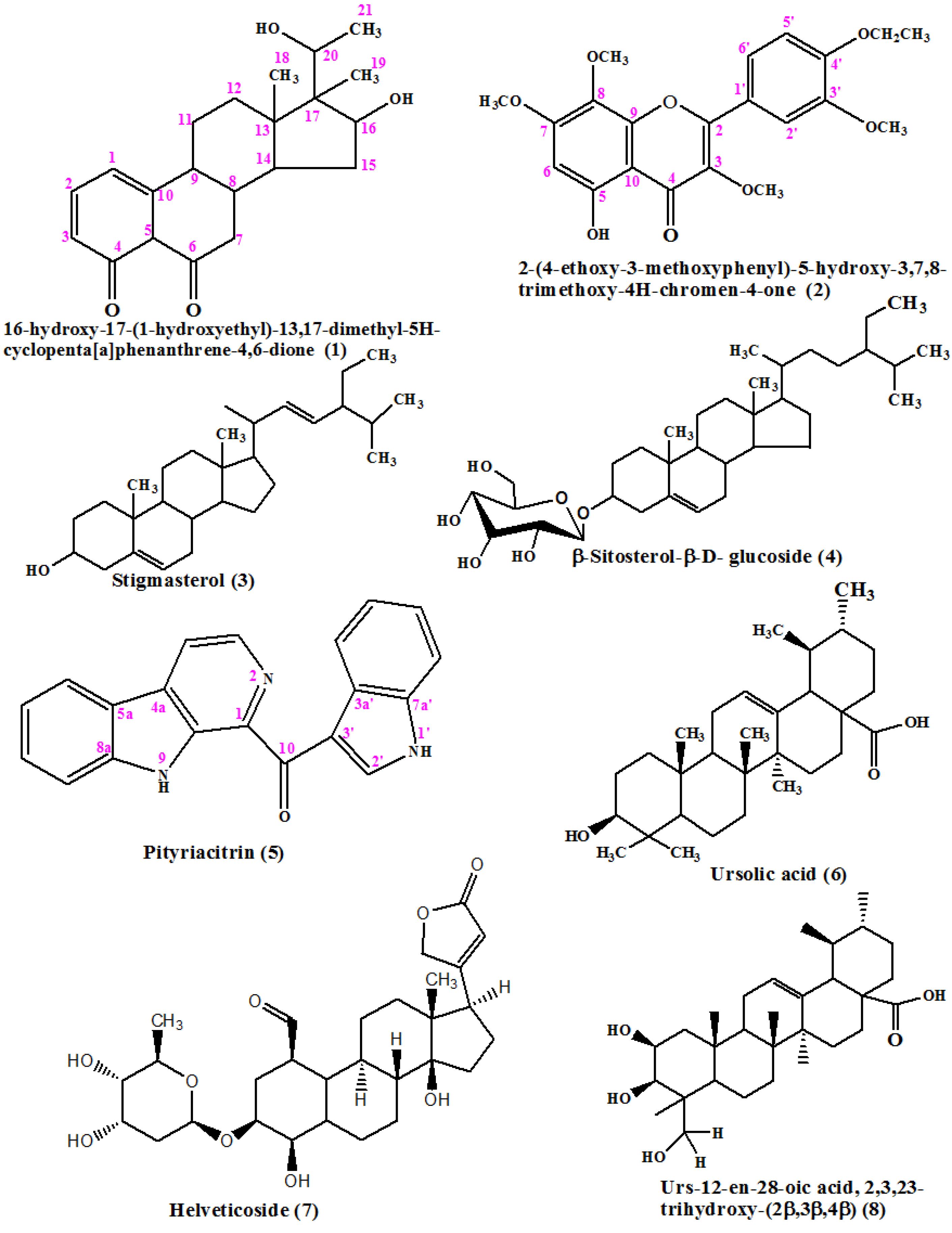
Figure 1.
Structures of compounds 1-8 from Strophanthus hispidus
.
Structures of compounds 1-8 from Strophanthus hispidus
In-depth spectroscopic analysis using 1D-(1H and 13C), 2D-NMR, low and high-resolution EI-MS led to the elucidation of the structures of the compounds. Comparison with existing literature of known compounds were checked for isolated compounds. The present study is the first report of the chemical investigation of cytotoxicity of S. hispidus.
Compound-1: 16-hydroxy-17-(1-hydroxyethyl)-13, 17-dimethyl-5H-cyclopenta[a]phenanthrene-4,6-dione: Off-white powder; EI-MS: m/z 344.2
corresponded to the molecular formula of C21H28O4; 1H and 13C NMR (Table 2)
Table 2.
NMR spectral assignment of compound 1 (ppm, CDCl3, 500 and 125 MHz)
|
Position
|
1H-NMR chemical shifts δ in ppm (coupling constant
J
in Hz)
|
13C-NMR chemical shifts δ in ppm / HSQC/carbon type
|
| 1 |
5.95 dd (1.5, 9.5) |
138.6, CH |
| 2 |
6.10 dd (2.5, 9.5) |
128.8, CH |
| 3 |
5.60 s |
124.1, CH |
| 4 |
-- |
199.3, Qc |
| 5 |
1.30 m |
48.7, CH |
| 6 |
-- |
215, Qc |
| 7 |
2.40 m
2.50 m |
33.8,CH2 |
| 8 |
2.10 m |
34.8, CH |
| 9 |
1.70 m |
57.7, CH |
| 10 |
-- |
162.6, Qc |
| 11 |
1.60 s |
27.6, CH2 |
| 12 |
1.99 m |
33.5, CH2 |
| 13 |
-- |
48.5, Qc |
| 14 |
3.40 d (4.0) |
64.2, Qc |
| 15 |
1.50 m |
29.5, CH2 |
| 16 |
4.0 m |
73.1, CH |
| 17 |
-- |
35.9, Qc |
| 18 |
1.07 s |
16.1, CH3 |
| 19 |
0.9 s |
10.4, CH3 |
| 20 |
4.50 dd (6.5, 16.0) |
65.7, CH |
| 21 |
1.05 s |
16.8, CH3 |
Note: s = singlet, t = triplet, J = coupling constant in Hz, q = quartet, d = doublet, dd = double doublet, m = multiplet. Qc = quaternary carbon.
IR (cm-1): 3341, 2946, 2871, 1651, 1053.
Melting point: 181.2 °C
Compound-2: 2-(4-ethoxy-3-methoxyphenyl)-5-hydroxy-3,7,8-trimethoxy-4H-chromen-4-one: Yellow powder; EI-MS: m/z 402.3 corresponded to the molecular formula of C21H22O8; 1H and 13C NMR (Table 3)
Table 3.
NMR spectral assignment for compound 2 in CDCl3 (500 and 125 MHz)
|
Position
|
1H-NMR
|
13C-NMR/ HSQC
|
HMBC
|
COSY
|
| 1 |
-- |
-- |
-- |
-- |
| 2 |
-- |
155.9 |
-- |
-- |
| 3 |
3.85 s
-- |
60.2
138.8 |
138.8 |
|
| 4 |
-- |
178.8 |
-- |
|
| 5 |
12.59 s |
152.7 |
178.8, 155.9, 132.2, 106.6 |
|
| 6 |
6.48 s |
90.3 |
178.8, 152.3, 132.2, 106.6 |
|
| 7 |
3.94 s
-- |
56.1
158.7 |
158.7 |
|
| 8 |
3.90 s
-- |
60.8
132.2 |
132.2 |
|
| 9 |
-- |
132.3 |
-- |
|
| 10 |
-- |
106.6 |
-- |
|
| 1ˈ |
-- |
122.7 |
-- |
|
| 2ˈ |
7.67 dd (2.0, 3.6) |
111.5 |
150.8, 148.9, 122.1 |
|
3ˈ
3ˈˈ |
--
3.94 s |
148.9
56.3 |
148.9 |
|
| 4ˈ |
-- |
150.8 |
-- |
|
| 5ˈ |
6.97 d (8.4) |
111.8 |
150.8, 148.9, 122.7, 111.5 |
7.70 |
| 6ˈ |
7.70 d (2.0) |
122.1 |
150.8, 111.5 |
6.97 |
| 1ˈˈ |
4.21 q |
64.4 |
150.8, 14.6 |
1.50 |
| 2ˈˈ |
1.50 t (7.2) |
14.6 |
64.4 |
4.21 |
Note: s = singlet, t = triplet, J = coupling constant in Hz, q = quartet, d = doublet, dd = double doublet, HMBC = Heteronuclear Multiple Bond Correlation, COSY = Heteronuclear Single Quantum Coherence.
IR (cm-1): 3727, 3624, 2932, 1267, 1217
Melting point: 128.2 °C
The 1H and 13C NMR spectral assignments for compounds 3-9 were reported in the supplementary file as Tables S1 and S2.
Compound-1 was isolated as an off-white powder. The compound also elicits UV absorption at 220, 230 and 280 nm (Figure S1) which are the characteristic absorption bands of phenanthrene skeleton.33 The 220 and 230 bands indicate п- п* transition suggesting the presence of double bond-containing compounds. Also, 282 nm suggests the presence of n- п* transition found due to the presence of the hydroxyl groups in the compound. The infrared absorption peak at 3341 represents the hydroxyl group, 2946 and 2871 cm-1, are typical of C-H stretching vibration, 1651, represents C = O stretch which is typical of an α,β-unsaturated keto-system and 1053 is typical of C-O stretch (Figure S2).
The HMBC spectrum of compound-1 (Figure S3), showed a correlation of δH 5.60(H-3) with δC 128.8(C-2), which suggests the presence of a double bond at 1 and 2 positions; δH 4.50(H-20) correlates with δC 16.8(C-18), 73.1(C-16) and 65.7(C-20), this suggests the presence of oxo-methine carbon signals at position 20. A correlation was observed between δH 6.10(H-2) with δC 124.1(C-3) and 162.6(C-10) suggesting the presence of olefinic double bonds at positions 2, 3 and 10 respectively. Another proton signal at δH 4.0(H-16) was found to correlate with δC 73.1(C-16) and 65.7(C-20), suggesting the presence of an oxo-methine signal at position 16. Furthermore, δH 3.40(H-16) correlates with δC 29.5(C-15) and 65.7(C-20), suggesting the presence of another oxo-methine signal at position 20. The δH 0.90(H-19) linked with δC 48.5(C-13) and 73.1(C-16) infers a methyl group present at position 19 and a hydroxylated system at position 16. Additionally, δH 1.06(H-21) in association with δC 65.7(C-20) suggests a methyl group at position 21. A correlation between δH 1.07(H-16) and δC 35.9(C-17), 48.5(C-13), suggests a methyl group at position 18 while proton signal at δH 1.60(H-11) in relation to δC 33.5(C-12) and 57.7(C-9), suggests the presence of an sp2-hybridised methylene signals at positions 11 and 12 respectively (Figures S4, S5).
The 1H-1H COSY spectrum showed a correlation between δH 4.5(H-20) and δH 1.05(H-21), with another existing between δH 4.0(H-16) and δH 1.50(H-15). Also, δH 2.50(H-7) with δH 2.10(H-8), and then, δH 2.10(H-8) with δH 1.70(H-11) (Figure S6).
The EI-MS spectrum, (Figure S7) showed m/z 344.2 as the molecular ion peak which confirms the molecular mass of the compound. The Fast Atom Bombardment (FAB) mass spectrum (Figure S8) of Compound 1 showed a strong protonated molecular ion at m/z 345.2 as proof of the molecular weight of the compound. The Loss of a molecule of water in the compound gave m/z 326; which is a characteristic of hydroxyl-containing compounds. The [C2H6O] loss gave fragment ion [C19H22O3] + with m/z 298, followed by a loss of [OH] group to give [C19H23O2] + with m/z 283. A loss of a neutral molecule; [C10H19O2] from the molecular ion gave [C11H9O2] + and [C15H25O3] with m/z 173 and 91 respectively. Also, loss of [C8H5O2] from the molecular ion gave [C13H21O2] + with m/z 211. A loss of [C15H17O2] from the molecular ion peak gave fragment ion [C6H11O2] + with m/z 115, while another loss of [C5H12O2] from the molecular ion gave [C16H16O2] + with m/z 240; which confirms the base peak ion as illustrated in the proposed fragmentation mechanistic pathways in (Figure S9). This is the first time isolating 16-hydroxy-17-(1-hydroxyethyl)-13,17-dimethyl-5H-cyclopenta[a]phenanthrene-4,6-dione from nature.
A compound, 3β- Acetoxy-17-(3-pyridyl) androsta-5,16-diene (abiraterone acetate) with a similar phenanthrene skeleton as compound 1 has been previously synthesized. This drug was reported to be designed for the treatment of prostatic carcinoma which is hormone- dependent.34
Compound-2 was suggested to be a flavone based on its physico-chemical properties as well as its chromatography performance. Its UV absorption at 232, 257 nm is indicative of the presence of chromophoric group in the compound (Figure S10). Infra-red data showed IR (KBr) Vmax cm-1: 3727, 3624 (OH group present at C-5), 2932 (C-Hstretch), typical of the non-aromatic part of the compound, side chain C-1ˈˈ and C-2ˈˈ), 1654 (-C = O, 4-keto-3-ene group, presence of α,β unsaturated system which is typical of flavones, 1217 (C-Ostretch) (Figure S11).
Electron ionization- mass spectroscopy spectrum of the compound (Figure S12) showed m/z 402.3; confirming the molecular ion of the compound. The loss of a methyl group on the ethoxy part from the side chain gave m/z 371, in addition to the total loss of the ethoxy moiety, 35 resulting in m/z 357. The hydroxyl group loss with a methyl group from the molecular ion peak gave m/z 355.
The NMR data showed the presence of four methoxyl groups [δH 3.85(3H, s, OCH3-3), 3.90(3H, s, OCH3-8), 3.94(6H, s, OCH3-7 and 3ˈ, respectively), with the corresponding δC 60.2, 60.8, 56.1 and 56.3 respectively]. An ethoxy group [δH 4.21 (2H, q, H-1ˈˈ and a terminal CH3 δH 1.50(3H, t, H-2ˈˈ)], four aromatic protons [δH 6.48(1H, s, H-6), 6.92(1H, d, H-5ˈ), 7.67(1H, dd, H-2ˈ) and 7.70(1H, d, H-6ˈ), with the matching δC 90.3 (C-6), 111.8 (C-5ˈ), 111.5 (C-2ˈ) and 122.1 (C-6ˈ), respectively] were also observed. A hydroxyl proton δH 12.9 with eleven quaternary carbons (Figures S13 and S14) [158.7(C-7), 155.9(C-2), 152.7(C-5), 150.8(C-4ˈ), 148.9(C-3ˈ), 138.8(C-3), 132.3(C-9), 132.2(C-8), 122.7(C-1ˈ) and 106.6(C-10)] including a carbonyl carbon with δC 178.8 (C-4) were detected in the NMR data as shown (Table 3). The above-mentioned data suggested compound 2 to be a tetra- methoxyflavone flavone.
In the HMBC spectrum (Figure S15), correlations were observed between δH 3.85 and δC 138.8(C-3), δH 12.59 and δC 178.8(C-4)/ 155.9(C-2)/ 132.2(C-9), 106.6(C-10). This confirmed the position of the carbonyl carbon. Proton and carbon signals at δH 6.48 and δC 178.8(C-4)/152.3(C-5)/ 132.2(C-9)/106.6(C-10), δH 3.94 and δC 158.7(C-7), δH 3.90 and δC 132.2(C-9), δH 7.67 and δC 150.8(C-4ˈ)/148.9(C-3ˈ)/122.1(C-6ˈ), δH 6.97 and δC 150.8(C-4ˈ)/148.9(C-3ˈ)/122.7(C-1ˈ)/111.5(C-2ˈ), δH 7.70 and δC 150.8(C-4ˈ)/111.5(C-2ˈ), further confirmed the positions of the methoxy and hydroxyl groups on the flavone ring.
The 1H-1H-COSY correlations of Compound-2 (Figure S16) showed a strong correlation between two vicinal protons H-5ˈ (δH 6.97) and H-6ˈ (δH 7.70), also H-1ˈˈ (δH 4.21) and H-2ˈˈ (δH 1.50). This correlation further confirmed the presence of the ethoxy group at C-4ˈ, and the other two vicinal protons at C-5ˈ and C-6ˈ.
A previously reported compound, 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8 trimethoxy-4H-chromen-4-one (2a), C19H18O8,35 is analogous to our isolated new flavone (2), 2-(4-ethoxy-3-methoxyphenyl)-5-hydroxy-3,7,8-trimethoxy-4H-Chromen-4-one. The only observable difference in the structure of the two compounds is at position 4ˈ, where the OH- group in (2a) is substituted by an ethoxy (OCH2CH3) group in (2) thus, further confirming an increase of 29 g/mol in molecular mass of the compound-2
All isolated compounds were subjected to cytotoxic assay as shown in Table 4. After 72 hours of treatment, compounds 1, 4, 6, 7, and 8, exhibited significant cytotoxic effects against breast and or skeletal muscle cancer cell lines.
Table 4.
Cytotoxicity of isolated compounds against cancer and normal cell lines
|
Compound
|
AU565
|
RD
|
MCF-7
|
3T3
|
|
IC50
±SEM (µM)
|
IC50
±SEM (µM)
|
IC50
±SEM (µM)
|
IC50
±SEM (µM)
|
| 1 |
NT |
16.49 ± 0.22 |
18.81 ± 0.29 |
NT |
| 2 |
NT |
69.01 ± 0.14 |
73.24 ± 0.03 |
NT |
| 3 |
25.00 ± 0.03 |
NT |
NT |
Not cytotoxic |
| 4 |
10.71 ± 0.12 |
34.28 ± 0.02 |
42.54 ± 0.45 |
Not cytotoxic |
| 5 |
29.29 ± 2.90 |
25.28 ± 1.06 |
41.75 ± 0.43 |
Not cytotoxic |
| 6 |
8.07 ± 0.02 |
0.08 ± 0.0# |
3.17 ± 0.26 |
Not cytotoxic |
| 7 |
11.42 ± 0.60 |
46.89 ± 0.05 |
20.57 ± 1.28 |
Not cytotoxic |
| 8 |
18.27 ± 1.73 |
3.15 ± 0.37 |
38.57 ± 0.45 |
Not cytotoxic |
| Doxorubicin* |
0.08 ± 0.04 |
-- |
-- |
-- |
| Vincristine |
-- |
0.41 ± 0.01 |
0.89 ± 0.12 |
-- |
| Cycloheximide* |
-- |
-- |
-- |
0.8 ± 0.1 |
AU565: Breast adenocarcinoma; RD: Rhabdomyosarcoma; MCF-7: Breast adenocarcinoma; NT: Not tested. *Standard drug for the study; # The mean difference is significant at P < 0.05 with respect to standard drug.
The cytotoxicity of Compound-1showed a relationship with two compounds with the Phenanthrene skeleton isolated from Asarum heterotropoides which displayed a strong cytotoxicity against Human renal proximal tubular epithelial cell lines (HK-2).36 Also, the new compound-1,showed IC50 of value of 16.49, 18.81 µM against RD and MCF-7.
Ursolic acid exhibited a significant IC50 values of 0.08, 3.17 and 8.07 µM against AU565, RD, and MCF-7 cancer cell lines respectively. The isolated Ursolic acid and helveticoside had great and promising cytotoxic properties on breast adenocarcinoma, AU565 with less cytotoxic effect on normal cells as shown in Table 4. Studies have shown that ursolic acid isolated from Dipterocarpus obtusifolius37 and Juglans regia L.38 had a great cytotoxic effect against MCF-7 cells. Ursolic acid has been reported useful in the management of cancer by inhibition of the tumour growth and spread using the B16 mouse melanoma-treated C57BL/6 mice in-vitro39; the expression of NF-kB activating effect of ursolic acid on melanoma, neuroblastoma cancer cell lines was decreased.40 The cytotoxic effect of ursolic acid was illustrated by apoptosis, a mechanism of action in Molt4, U937 and K562 leukaemia cell,41 which is in line with the in vivo inhibitory assay of aberrant crypt foci, a precursor of colon cancer showed ursolic acid exhibiting chemotherapeutic effects.42 Based on findings in existing literature, this is the first time of isolation of ursolic acid from S. hispidus though isolation of its methyl ester was reported from S. speciosus.24 Secondary metabolites such as triterpenes has promising cytotoxic properties for the treatment of cancer with different modes of action.43
From this study, helveticoside showed strong activity with IC50 of 11.42 µM against the AU565 cell line. To the best of our knowledge, this is the first report of helveticoside from S. hispidus. Reports have shown cytotoxicity of helveticoside with a strong IC50 range of between 0.034 and 0.596 µM on RAW264.7, HepG2, HCT116, SK-OV-3.29 Helveticoside isolated from Descurainia sophia showed induction of gene regulation in A549, human lung cancer cell line.44
A previous isolation of pityriacitrin from S. hispidus has not been reported in the literatureA study on pityriacitrin by Zhang et al showed minimal cytotoxic activity against breast (MDA-231 and MCF-7) and prostrate carcinoma (PC3) in agreement with observation in this study.45
The inhibition of the cell growth of AU565 breast carcinoma by daucosterol was observed in this study which is correlated with an earlier report on the significant effect of the compound on breast cancer apoptosis and metastasis.36 Its cytotoxic effects on prostate cancer cells, DU145 and PC3 with IC50 of 29.25 and 17.45 µg/mL respectively46 and against lung carcinoma, A54947 were also reported.
In line with this study, bioactive compounds have pharmacophoric characteristics in common with medications that are now prescribed to treat several illnesses.48 Natural products offer a wealth of potentially appealing scaffolds and molecular building blocks for synthesis.49 With this, molecular modification can be used to alter the chemical structure of natural compounds because they have not been developed as medicines. This will accomplish ideal pharmacokinetic and pharmacodynamic qualities or improve efficacy and selectivity for the target, breast cancer, as in this case.
Conclusion
Strophanthus hispidus has shown to be of high potential with hope in the management of cancer. Significant cytotoxicity and selectivity of S. hispidus were observed at all stages of isolation of the compounds, therefore, showing little or no toxicity. Hence ursolic, urs-12-en-28-oic acid, 2, 3, 23-trihydroxy-(2β, 3β, 4α) and helveticoside can be developed as a safe and effective pharmaceutical composition against breast adenocarcinoma as a means of inducing cell death, apoptosis. This study, therefore, justifies the claims by traditional medicinal practitioners of the effectiveness of the S. hispidus in the management of cancer in South West Nigeria.
Competing Interests
The authors declare no conflict of interest.
Supplementary Files
The spectral assignments for compounds 3-9 were reported in the supplementary file as Tables S1 and S2 and Figure S1-S16.
(pdf)
Acknowledgements
The authors sincerely appreciate The World Academy of Science-International Centre for Chemical and Biological Science (TWAS-ICCBS) for providing fellowship to Owoola Azeezat AMBALI. The HEJ and PCMB institutes are greatly appreciated for the facilities for isolation, characterization and also screening of the plant and isolated compounds, respectively. The authors are grateful to Ms. Anam, Dr. Rehan (ICCBS) and Mr Toluwanimi Akinleye (Pharmacognosy Department, University of Ibadan) for the cytotoxicity assay. We acknowledge the staff of the Forest Herbarium Ibadan (FHI) for the identification and authentication of plants.
References
- Ezuruike UF, Prieto JM. The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. J Ethnopharmacol 2014; 155(2):857-924. doi: 10.1016/j.jep.2014.05.055 [Crossref] [ Google Scholar]
- Ugboko HU, Nwinyi OC, Oranusi SU, Fatoki TH, Omonhinmin CA. Antimicrobial importance of medicinal plants in Nigeria. ScientificWorldJournal 2020; 2020:7059323. doi: 10.1155/2020/7059323 [Crossref] [ Google Scholar]
- Brenes Bermúdez FJ, Alcántara Montero A. [Early detection or screening in the prevention of prostate cancer?]. Semergen 2017; 43(2):100-8. doi: 10.1016/j.semerg.2016.01.014.[Spanish] [Crossref] [ Google Scholar]
- Lim EJ, Torresi J. Prevention of hepatitis C virus infection and liver cancer. Recent Results Cancer Res 2021; 217:107-40. doi: 10.1007/978-3-030-57362-1_6 [Crossref] [ Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021; 71(1):7-33. doi: 10.3322/caac.21654 [Crossref] [ Google Scholar]
- Shankar A, Dubey A, Saini D, Singh M, Prasad CP, Roy S. Environmental and occupational determinants of lung cancer. Transl Lung Cancer Res 2019; 8(Suppl 1):S31-49. doi: 10.21037/tlcr.2019.03.05 [Crossref] [ Google Scholar]
- Tran KB, Lang JJ, Compton K, Xu R, Acheson AR, Henrikson HJ. The global burden of cancer attributable to risk factors, 2010-19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022; 400(10352):563-91. doi: 10.1016/s0140-6736(22)01438-6 [Crossref] [ Google Scholar]
- Delahunty R, Nguyen L, Craig S, Creighton B, Ariyaratne D, Garsed DW. TRACEBACK: testing of historical tubo-ovarian cancer patients for hereditary risk genes as a cancer prevention strategy in family members. J Clin Oncol 2022; 40(18):2036-47. doi: 10.1200/jco.21.02108 [Crossref] [ Google Scholar]
- Walsh MF, Cadoo K, Salo-Mullen EE, Dubard-Gault M, Stadler ZK, Offit K. Genetic factors: hereditary cancer predisposition syndromes. In: Niederhuber JE, Armitage JO, Kastan MB, Doroshow JH, Tepper JE, eds. Abeloff’s Clinical Oncology. 6th ed. Philadelphia: Elsevier; 2020. p. 180-208.e11. doi: 10.1016/b978-0-323-47674-4.00013-x.
- Kyprianou G. Cancer: cancer in the 21st century, the role of lifestyle and nutrition, food controversies and recommendations for cancer prevention: article. Act Sci Nutr Health 2019; 3(7):173-8. [ Google Scholar]
- Fatiregun OA, Bakare O, Ayeni S, Oyerinde A, Sowunmi AC, Popoola A. 10-year mortality pattern among cancer patients in Lagos State University Teaching Hospital, Ikeja, Lagos. Front Oncol 2020; 10:573036. doi: 10.3389/fonc.2020.573036 [Crossref] [ Google Scholar]
- Ahmad R, Khan MA, Srivastava AN, Gupta A, Srivastava A, Jafri TR. Anticancer potential of dietary natural products: a comprehensive review. Anticancer Agents Med Chem 2020; 20(2):122-236. doi: 10.2174/1871520619666191015103712 [Crossref] [ Google Scholar]
- Zhang B, Zhang T, Xu J, Lu J, Qiu P, Wang T. Marine sponge-associated fungi as potential novel bioactive natural product sources for drug discovery: a review. Mini Rev Med Chem 2020; 20(19):1966-2010. doi: 10.2174/1389557520666200826123248 [Crossref] [ Google Scholar]
- Kumar A, Patil D, Rajamohanan PR, Ahmad A. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS One 2013; 8(9):e71805. doi: 10.1371/journal.pone.0071805 [Crossref] [ Google Scholar]
- Yamamoto K, Takahashi K, Caputi L, Mizuno H, Rodriguez-Lopez CE, Iwasaki T. The complexity of intercellular localisation of alkaloids revealed by single-cell metabolomics. New Phytol 2019; 224(2):848-59. doi: 10.1111/nph.16138 [Crossref] [ Google Scholar]
- Inokawa H, Katayama N, Nakao M. Evaluation of multidrug cancer chronotherapy based on cell cycle model under influences of circadian clock. Annu Int Conf IEEE Eng Med Biol Soc 2016; 2016:1439-42. doi: 10.1109/embc.2016.7590979 [Crossref] [ Google Scholar]
- Satheesh NJ, Samuel SM, Büsselberg D. Combination therapy with vitamin C could eradicate cancer stem cells. Biomolecules 2020; 10(1):79. doi: 10.3390/biom10010079 [Crossref] [ Google Scholar]
- Ambali OA, Ajaiyeoba EO, Ogbole OO, Adeniji JA. Ethnobotanical survey of plants used for cancer treatment in Akinyele local government of Ibadan, Nigeria and preliminary cytotoxic activity of selected plants. Niger J Pharm Res 2021; 17(1):27-37. doi: 10.4314/njpr.v17i1.3 [Crossref] [ Google Scholar]
- Burkill HM. The Useful Plants of West Africa. Vol 1. Royal Botanical Gardens; 1985. p. 386-7.
- Ayoola GA, Folawewo AD, Adesegun SA, Abioro OO, Adepoju-Bello AA, Coker HA. Phytochemical and antioxidant screening of some plants of Apocynaceae from South West Nigeria. Afr J Plant Sci 2008; 2(9):124-8. [ Google Scholar]
- Ojiako OA, Igwe CU. A time-trend hypoglycemic study of ethanol and chloroform extracts of Strophanthus hispidus. J Herbs Spices Med Plants 2009; 15(1):1-8. doi: 10.1080/10496470902787386 [Crossref] [ Google Scholar]
- Carvalho V, Fernandes L, Conde T, Zamith H, Silva R, Surrage A. Antinociceptive activity of Stephanolepishispidus skin aqueous extract depends partly on opioid system activation. Mar Drugs 2013; 11(4):1221-34. doi: 10.3390/md11041221 [Crossref] [ Google Scholar]
- Vardanyan RS, Hruby VJ. Cardiotonic drugs. In: Vardanyan RS, Hruby VJ, eds. Synthesis of Essential Drugs. Amsterdam: Elsevier; 2006. p. 237-43. doi: 10.1016/b978-044452166-8/50017-0.
- Emamzadeh-Yazdi S. Antiviral, Antibacterial and Cytotoxic Activities of South African Plants Containing Cardiac Glycosides [dissertation]. South Africa: University of Pretoria; 2013.
- Karkare S, Adou E, Cao S, Brodie P, Miller JS, Andrianjafy NM. Cytotoxic cardenolide glycosides of Roupellina (Strophanthus) boivinii from the Madagascar rainforest. J Nat Prod 2007; 70(11):1766-70. doi: 10.1021/np070336n [Crossref] [ Google Scholar]
- Ogbole O, Segun P, Akinleye T, Fasinu P. Antiprotozoal, antiviral and cytotoxic properties of the Nigerian mushroom, Hypoxylonfuscum Pers Fr (Xylariaceae). Acta Pharm Sci 2018; 56(4):43-56. doi: 10.23893/1307-2080.aps.05625 [Crossref] [ Google Scholar]
- Bray F, Parkin DM. Cancer in sub-Saharan Africa in 2020: a review of current estimates of the national burden, data gaps, and future needs. Lancet Oncol 2022; 23(6):719-28. doi: 10.1016/s1470-2045(22)00270-4 [Crossref] [ Google Scholar]
- Chaturvedula VS, Prakash I. Isolation of stigmasterol and ?-Sitosterol from the dichloromethane extract of Rubus suavissimus. Int Curr Pharm J 2012; 1(9):239-42. doi: 10.3329/icpj.v1i9.11613 [Crossref] [ Google Scholar]
- Lee JM, Lee DG, Lee KH, Cho SH, Nam KW, Lee S. Isolation and identification of phytochemical constituents from the fruits of Acanthopanax senticosus. Afr J of Pharm Pharmacol 2013; 7(6):294-301. doi: 10.5897/ajpp12.898 [Crossref] [ Google Scholar]
- Mayser P, Schäfer U, Krämer HJ, Irlinger B, Steglich W. Pityriacitrin--an ultraviolet-absorbing indole alkaloid from the yeast Malassezia furfur. Arch Dermatol Res 2002; 294(3):131-4. doi: 10.1007/s00403-002-0294-2 [Crossref] [ Google Scholar]
- Nakamura T, Goda Y, Sakai S, Kondo K, Akiyama H, Toyoda M. Cardenolide glycosides from seeds of Corchorus olitorius. Phytochemistry 1998; 49(7):2097-101. doi: 10.1016/s0031-9422(98)00421-x [Crossref] [ Google Scholar]
- Khine MM. Isolation and Characterization of Phytoconstituents from Myanmar Medicinal Plants [dissertation]. Wittenberg: Der Martin Luther Universität Halle. 2006.
- Jing Y, Zhang YF, Shang MY, Yu J, Tang JW, Liu GX. Phenanthrene derivatives from roots and rhizomes of Asarum heterotropoides var mandshuricum. Fitoterapia 2017; 117:101-8. doi: 10.1016/j.fitote.2017.01.008 [Crossref] [ Google Scholar]
- Potter GA, Hardcastle IR, Jarman M. A convenient, large-scale synthesis of Abiraterone acetate [3β-acetoxy-17-(3-pyridyl) androsta-5, 16-diene], a potential new drug for the treatment of prostate cancer. Org Prep Proced Int 1997; 29(1):123-8. doi: 10.1080/00304949709355175 [Crossref] [ Google Scholar]
- Brito I, Bórquez J, Simirgiotis M, Cárdenas A. Crystal structure of 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,7,8-trimethoxy-4 H-chromen-4-one, C19H18O8. Z Kristallogr New Cryst Struct 2018; 233(1):61-4. doi: 10.1515/ncrs-2017-0141 [Crossref] [ Google Scholar]
- Han B, Jiang P, Liu W, Xu H, Li Y, Li Z. Role of daucosterol linoleate on breast cancer: studies on apoptosis and metastasis. J Agric Food Chem 2018; 66(24):6031-41. doi: 10.1021/acs.jafc.8b01387 [Crossref] [ Google Scholar]
- Khiev P, Kwon OK, Song HH, Oh SR, Ahn KS, Lee HK. Cytotoxic terpenes from the stems of Dipterocarpus obtusifolius collected in Cambodia. Chem Pharm Bull (Tokyo) 2012; 60(8):955-61. doi: 10.1248/cpb.c12-00012 [Crossref] [ Google Scholar]
- Tsasi G, Samara P, Tsitsilonis O, Jürgenliemk G, Skaltsa H. Isolation, Identification and cytotoxic activity of triterpenes and flavonoids from green walnut (Juglans regia L) pericarps. Rec Nat Prod 2016; 10(1):83-92. [ Google Scholar]
- Lee I, Lee J, Lee YH, Leonard J. Ursolic acid-induced changes in tumor growth, O2 consumption, and tumor interstitial fluid pressure. Anticancer Res 2001; 21(4A):2827-33. [ Google Scholar]
- Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res 2003; 63(15):4375-83. [ Google Scholar]
- Urech K, Scher JM, Hostanska K, Becker H. Apoptosis inducing activity of viscin, a lipophilic extract from Viscum album L. J Pharm Pharmacol 2005; 57(1):101-9. doi: 10.1211/0022357055083 [Crossref] [ Google Scholar]
- Andersson D, Cheng Y, Duan RD. Ursolic acid inhibits the formation of aberrant crypt foci and affects colonic sphingomyelin hydrolyzing enzymes in azoxymethane-treated rats. J Cancer Res Clin Oncol 2008; 134(1):101-7. doi: 10.1007/s00432-007-0255-4 [Crossref] [ Google Scholar]
- Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med 1995; 1(10):1046-51. doi: 10.1038/nm1095-1046 [Crossref] [ Google Scholar]
- Kim BY, Lee J, Kim NS. Helveticoside is a biologically active component of the seed extract of Descurainiasophia and induces reciprocal gene regulation in A549 human lung cancer cells. BMC Genomics 2015; 16(1):713. doi: 10.1186/s12864-015-1918-1 [Crossref] [ Google Scholar]
- Zhang JA, Xuan T, Parmar M, Ma L, Ugwu S, Ali S. Development and characterization of a novel liposome-based formulation of SN-38. Int J Pharm 2004; 270(1-2):93-107. doi: 10.1016/j.ijpharm.2003.10.015 [Crossref] [ Google Scholar]
- Zingue S, Gbaweng Yaya AJ, Michel T, Ndinteh DT, Rutz J, Auberon F. Bioguided identification of daucosterol, a compound that contributes to the cytotoxicity effects of Cratevaadansonii DC (Capparaceae) to prostate cancer cells. J Ethnopharmacol 2020; 247:112251. doi: 10.1016/j.jep.2019.112251 [Crossref] [ Google Scholar]
- Rajavel T, Mohankumar R, Archunan G, Ruckmani K, Devi KP. Beta sitosterol and daucosterol (phytosterols identified in Grewia tiliaefolia) perturbs cell cycle and induces apoptotic cell death in A549 cells. Sci Rep 2017; 7(1):3418. doi: 10.1038/s41598-017-03511-4 [Crossref] [ Google Scholar]
- Islam F, Khadija JF, Harun-Or-Rashid M, Rahaman MS, Nafady MH, Islam MR. Bioactive compounds and their derivatives: an insight into prospective phytotherapeutic approach against Alzheimer’s disease. Oxid Med Cell Longev 2022; 2022:5100904. doi: 10.1155/2022/5100904 [Crossref] [ Google Scholar]
- Meng G, Hu L, Chan HSS, Qiao JX, Yu JQ. Synthesis of 1,3-dienes via ligand-enabled sequential dehydrogenation of aliphatic acids. J Am Chem Soc 2023; 145(24):13003-7. doi: 10.1021/jacs.3c03378 [Crossref] [ Google Scholar]