Pharmaceutical Sciences. 2025;31(3):251-260.
doi: 10.34172/PS.025.40918
Research Article
Exploring the Immunomodulatory Effects of Liposomal Daunorubicin on Jurkat Cells: A Study of Apoptosis and Gene Expression in Co-Cultured MDA-MB-231 Cells
Malik Kareem Shanan Data curation, Formal analysis, Funding acquisition, Methodology, Resources, Software, Visualization, Writing – original draft, 1 
Amir Arasteh Formal analysis, Validation, 2 
Mahsa Mohamad Amoli Formal analysis, Validation, 3 
Mehdi Ebrahimi Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing, 4, * 
Author information:
1Department of Cell and Molecular Biology, Faculty of Converging Sciences and Technologies, Science and Research Branch, Islamic Azad University, Tehran, Ira
2Department of Biology, Faculty of Sciences, Rasht Branch, Islamic Azad University, Rasht, Iran
3Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Iran
4Department of Biochemistry and Biophysics, College of Biological Sciences, Varamin-Pishva Branch, Islamic Azad University, Pishva, Iran
Abstract
Background:
Breast cancer represents a significant aspect of women’s health, constituting approximately 30% of cancers affecting them globally, with a mortality rate of around 15%. In the quest for healing, immunotherapy has emerged as a beacon of hope, employing our body’s innate defenses to confront cancer. Cytotoxic T lymphocyte-associated protein 4 (CTLA-4) acts as a guardian, regulating immune responses, while COX-1’s influence on inflammation and invasion in cancer is modest yet notable. This study aims to explore the immunomodulatory effects of liposomal daunorubicin (LipDau) on Jurkat cells, evaluating the cancer cell fate through apoptosis and the expression of CTLA-4 and COX-1 genes in MDA-MB-231 cells as they co-exist with Jurkat cells.
Methods:
We assessed cell cytotoxicity in MDA-MB-231 cells under various treatments using the MTT assay. The apoptosis process was traced through flow cytometry, comparing controls, LipDau treatments, Jurkat cells, and activated Jurkat cells. The expression of CTLA-4 and COX-1 genes in MDA-MB-231 cells was measured through real-time PCR across similar treatments.
Results:
The application of LipDau, particularly in the presence of activated Jurkat cells, led to a significant decline in MDA-MB-231 cells, correlating with LipDau concentrations. Activated Jurkat cells demonstrated a lower IC50 than LipDau (21.5 and 31.1 μM, respectively). Notably, apoptosis rates rose sharply in MDA-MB-231 cells co-cultured with Jurkat cells at IC50 concentrations of LipDau, accompanied by decreased expression of CTLA-4 and COX-1 genes following treatment.
Conclusion:
Through the nurturing guidance of LipDau-activated Jurkat cells, a protective response emerged against tumor cells in MDA-MB-231, moderated by the CTLA-4 checkpoint and a suppression of inflammatory pathways via COX-1. These interactions facilitated the journey towards apoptosis and a halt in the relentless spread of metastatic breast cancer.
Keywords: Cancer, Breast cancer, Daunorubicin, Liposomes, Apoptosis, Immunomodulation
Copyright and License Information
© 2025 The Author(s).
This is an open access article and applies the Creative Commons Attribution Non-Commercial License (
http://creativecommons.org/licenses/by-nc/4.0/). Non-commercial uses of the work are permitted, provided the original work is properly cited.
Funding Statement
This study was performed as the M.Sc. thesis of the first author and no additional funding was used.
Introduction
Breast cancer remains one of the most pressing health concerns for women worldwide, accounting for approximately 30% of female cancer diagnoses and a mortality rate approaching 15%.1 The complexity of breast cancer manifests not only in its diverse biological subtypes but also in the challenges posed by its treatment, recurrence, and resistance.2 Standard treatments such as surgery, radiation, and traditional chemotherapy have provided avenues for recovery; however, they often come with significant side effects and may fail to completely eradicate the disease. This has led to an intensified search for innovative therapeutic strategies that harness the body’s own immune system, a burgeoning field known as cancer immunotherapy.3
Immunotherapy, particularly the use of checkpoint inhibitors and other immunomodulators, has shown promise in stimulating the immune system to recognize and eliminate cancer cells.4 Immunotherapy, particularly the use of checkpoint inhibitors and other immunomodulators, has shown promise in stimulating the immune system to recognize and eliminate cancer cells.5 Immunomodulation aims to enhance the immune response by activating the host immune system. This is done by stimulating antigen presenting cells to activate T cells, leading to the destruction of tumor cells by T cells. Cytokines are key agents in immunomodulation, with several cytokine-based immunomodulators approved for use in cancer immunotherapy 6. Another type of immunomodulator used in cancer treatment is checkpoint inhibitors. These inhibitors work by blocking certain proteins that stop the immune system from attacking cancer cells. They can also be considered a form of targeted therapy since they specifically target proteins on either T cells or cancer cells.
Recent studies have found that certain chemotherapy drugs like doxorubicin (Dox) can activate the immune system of the patient, either when used on their own or in combination with immunotherapy.5 Recently, liposomal formulations have emerged as a means to enhance the delivery and efficacy of chemotherapeutic agents, allowing for targeted action with reduced systemic toxicity. Liposomal daunorubicin (LipDau), an encapsulated form of the anthracycline daunorubicin, combines the advantages of traditional chemotherapy with the potential for immunomodulatory effects. By utilizing the innate properties of T cells, particularly CD4+T cells represented by Jurkat cells, LipDau may influence the tumor microenvironment and enhance anti-tumor immunity.7
Among various immune regulatory molecules, cytotoxic T lymphocyte-associated protein 4 (CTLA-4) has emerged as a prominent target in immunotherapy.8 CTLA-4 is expressed in many cell lines from variety of solid tumours and so tumour cell‐intrinsic CTLA-4 may play a role in tumorigenesis.9 As a negative checkpoint regulator, CTLA-4 plays a critical role in modulating T-cell activation and maintaining immune tolerance, thereby preventing potential damage to healthy tissues.10 However, tumor cells exploit this mechanism to evade immune detection, which underscores the necessity of targeting CTLA-4 to enhance anti-tumor responses.11 Concurrently, cyclooxygenase-1 (COX-1) is implicated in cancer-related inflammation and has been shown to influence tumor progression and immune interaction.12 The bioactive lipid prostaglandin E2 (PGE2) produced by COX-1 and COX-2 enzymes in cancer cells play a pivotal role in activation of cancer-specific CD8 + T cells and so anti-cancer immunity.13
In this study, we aimed to investigate the immunomodulatory effects of liposomal daunorubicin (LipDau) on Jurkat cells, a CD4+ T cell line. We hypothesize that LipDau will enhance the immune response against breast cancer cells by stimulating Jurkat cells to induce apoptosis in MDA-MB-231, an invasive breast tumor cell line. Furthermore, we will assess the expression of CTLA-4 and COX-1 genes in MDA-MB-231 cells to elucidate the underlying immunomodulatory mechanisms of LipDau. This research aims to contribute to the understanding of how LipDau can be utilized as a potential therapeutic strategy in breast cancer treatment.
Methods
Cell culture
MDA-MB-231 cells, a widely used model metastatic breast cancer cell line, were cultured in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM-F12) medium. CD4+ Jurkat cells, an immortalized T lymphocyte cell line, were maintained in RPMI 1640 medium, which is specifically designed for the growth of lymphocytes. The culture media for both cells were supplemented with 10% heat-inactivated fetal bovine serum (FBS). For the Jurkat cells, the RPMI 1640 medium was additionally supplemented with 1% antibiotic-antimycotic solution to prevent bacterial and fungal contamination, as well as 1% L-glutamine. Both cell lines were cultured under sterile conditions and were routinely observed daily using an inverted microscope to monitor their growth and morphology mycoplasma contamination, allowing for early detection of any changes in cell behavior or viability. The culture media were changed every 3 days to provide fresh nutrients and to remove waste products from the culture environment, which helps maintain optimal growth conditions. All cell cultures were maintained in a 37 °C humidified incubator with 5% CO2 to create an optimal environment, thereby ensuring the viability and functionality of both cell lines throughout the experiments.14
Preparation and characterization of LipDau
As reported in our published article,15 LipDau was synthesized using the thin-layer hydration method, following the procedure described by Chen et al16 The resulting liposomes, spherical in shape and measuring 50 to 100 nm in diameter, were analyzed using field emission scanning electron microscopy (FE-SEM) to determine their shape and size.
Cytotoxicity assay
Cytotoxicity was evaluated using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. MDA-MB-231 cells (5 × 10³ cells/well) were seeded in 96-well plates and incubated for 24 hours at 37 °C in a 5% CO2 atmosphere to allow to allow for cell attachment and growth to reach approximately 70% confluence. Subsequently, the culture medium was carefully aspirated and replaced with treatment solutions tailored to investigate both the effects of LipDau and the interaction between MDA-MB-231 and CD4+ Jurkat cells. In the LipDau treatment group, MDA-MB-231 cells received varying concentrations of LipDau (0, 15, 30, and 60 μM) to evaluate its dose-dependent cytotoxic effects. Each concentration of LipDau was prepared based on methods detailed in our previous study15 and was diluted in the culture medium to ensure even distribution and accurate dosing.
In the co-culture setting, the Jurkat cells were pre-treated for 24 hours with various concentrations LipDau (0, 15, 30, and 60 μm) for 24 hours under the same culture conditions.
Following treatment, 100 μL of MTT solution (5 mg/mL in PBS) was added to each well to quantify viable cells. The plates were then incubated for 3 hours at 37 °C to allow sufficient time for the reaction to occur. Next, the culture medium was carefully removed, and 100 μL of dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals formed within the viable cells. This dissolution step was followed by a 15 minutes at 37 °C to ensure complete solubilization. Finally, the optical density of each well was measured at 570 nm using a microplate reader. This was accomplished by dividing the absorbance of the treated samples by the absorbance of the control (untreated) wells and multiplying by 100 to express cell viability as a percentage.
IC50 values were determined by plotting the dose-response curves and fitting the data to a four-parameter logistic model using GraphPad Prism (version 10.4). The equation used was: Y = Bottom + (Top - Bottom) / [1 + 10^((LogIC50 - X) × HillSlope)], where Y is the measured response, X is the logarithm of the dose, ‘Top’ and ‘Bottom’ represent the plateaus of the curve, and the HillSlope describes the steepness of the curve. The goodness of the fit was evaluated by the R2 value.
Cell treatments
Co-culture experiments were performed in 6-well plates to assess the immunomodulatory interactions between Jurkat cells and MDA-MB-231 cells. The experimental groups included: (1) Control (MDA-MB-231 cells only), (2) LipDau (MDA-MB-231 cells treated with the IC50 concentration of LipDau), (3) Jurkat (MDA-MB-231 cells co-cultured with Jurkat cells), and (4) Jurkat-LipDau (MDA-MB-231 cells co-cultured with Jurkat cells, with Jurkat pre-treated with the IC50 concentration of LipDau). Jurkat cells were first cultured in a 6-well plate for 24 hours in medium containing the IC50 concentration of LipDau. This treatment was intended to enhance the cytotoxic potential of Jurkat cells by exposing them to an immune-modulating agent, thereby priming them for interaction with the breast cancer cells. Concurrently, MDA-MB-231 cells (1 × 10⁵ cells/well) were then seeded in separate 6-well plates and allowed to adequate cell adherence and growth for 24 hours. Following this initial incubation, the previously treated Jurkat cells (1 × 10⁵ cells/well) were introduced into the wells containing the MDA-MB-231 cells. The co-cultures were maintained for an additional 24 hours, facilitating direct cell-to-cell interactions between the Jurkat and MDA-MB-231 cells. After co-culture period, suspended Jurkat cells were gently removed. The residual MDA-MB-231 cells then incubated for an extended period of 72 hours to assess changes in cell viability and apoptosis following the immune interactions. Following the extended incubation, MDA-MB-231 cells were harvested for further analysis, specifically evaluating apoptosis and quantitative polymerase chain reaction (qPCR).
Flow cytometric analysis of apoptosis
Apoptosis in MDA-MB-231 cells from the various treatment groups was assessed using flow cytometry. Initially, MDA-MB-231 cells (1 × 10⁵ cells) from each treatment group were harvested and washed twice with phosphate-buffered saline (PBS) to remove any residual culture medium and to prepare them for staining. For the detection of apoptotic cells, we employed the Annexin-V-FITC Apoptosis Detection Kit (Sigma, Germany), which is specifically designed to identify early and late stages of apoptosis. According to the manufacturer’s instructions, the harvested cells were resuspended in a binding buffer at a concentration of 1 × 106 cells/mL. We then added 5 µL of Annexin V-FITC and 10 µL of propidium iodide (PI) to the cell suspension. The PI stain serves as a vital dye that cannot penetrate the intact membranes of living cells, thus allowing for the discrimination between viable, early apoptotic (Annexin V positive, PI negative), late apoptotic (Annexin V positive, PI positive), and necrotic cells (Annexin V negative, PI positive). The cells were gently mixed and incubated for 15 minutes in the dark at room temperature to ensure optimal staining. After the incubation period, 400 µL of binding buffer was added to each sample to dilute the cells before they were analyzed by flow cytometry. Flow cytometric analysis was conducted using a BD Biosciences instrument (Belgium). Data from the flow cytometric analysis were collected and analyzed using BD FACSDiva software, allowing for the quantification of each cell population. The results were presented as a percentage of total cell count per treatment group, enabling us to effectively compare the level of apoptosis among the different experimental conditions.
Quantitative PCR
To investigate the immunomodulatory mechanisms of LipDau on Jurkat cells, we evaluated the expression levels of key markers, specifically CTLA-4 and COX-1 genes in MDA-MB-231 cells. The first step in this process involved isolating RNA from the treated MDA-MB-231 cells using Total RNA was extracted from MDA-MB-231 cells using the TRIzol method.15 Following the manufacturer’s instructions, the cells were lysed in TRIzol reagent, and the RNA was purified through a series of chloroform extractions and isopropanol precipitations. The integrity and concentration of the isolated RNA were assessed using a spectrophotometer, ensuring that the RNA was suitable for downstream applications. Subsequently, complementary DNA (cDNA) was synthesized from the isolated RNA with the Easy cDNA Synthesis Kit (ParsTos, Iran). For the qPCR analysis, specific primer sets for target genes were designed and obtained from SinaClon (Iran). The primers included: CTLA4 (F: 5’ GGCAACCTACATGATGGGGAA 3’, R: 5’ GTATGGCGGTGGGTACATGAG 3’), COX-1 (F: 5’ GACCTGGCTCCGGAATTCACT 3’, R: 5’ ATGTGCTGAGTTGTAGGTGGG 3’), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; F: 5’ CTTTGGTATCGTGGAAGGAC 3’, R: 5’ GCAGGGATGATGTTCTGG 3’). GAPDH was chosen as the reference gene. The qPCR reactions were performed using a StepOneTM Real-Time PCR System (Thermo Fisher Scientific, USA). Each reaction mixture contained the synthesized cDNA, specific primers, and the Add SYBR Master mix from AddBio (South Korea). The relative expression levels of the target genes were quantified using the ∆∆CT method.15
Statistical analysis
All experiments were performed in triplicate to ensure the reliability and reproducibility of the results, allowing for a robust statistical evaluation of the data. The results from each experiment were presented as mean ± standard deviation (SD). Statistical analyses were conducted using GraphPad PRISM software (USA). The normality of data was assessed using the Kolmogorov-Smirnov test. For datasets that met the criteria for normal distribution, we employed one-way ANOVA test. Following the ANOVA, post hoc tests, such as Tukey’s test, applied to identify specific group differences if the ANOVA results indicated significance. Statistically significance was determined using a threshold of P < 0.05.
Results
MDA-MB-231 cells morphological assesment
The untreated and co-cultured with Jurkat cells MDA-MB-231 cells maintained their original epithelial-like spindle shaped morphology (Figure 1A and 1B, respectively). In contrast, the MDA-MB-231 cells lost their original shape by LipDau treatment (Figure 1C, 1G and 1E) and co-culture with activated Jurkat cells (Figure 1D, 1F and 1H). The MDA-MB-231 cells had lost their elongated spindle-shape morphology and suspension cells (dead cells) were identified. The number of dead cells were increased with increment in LipDau.
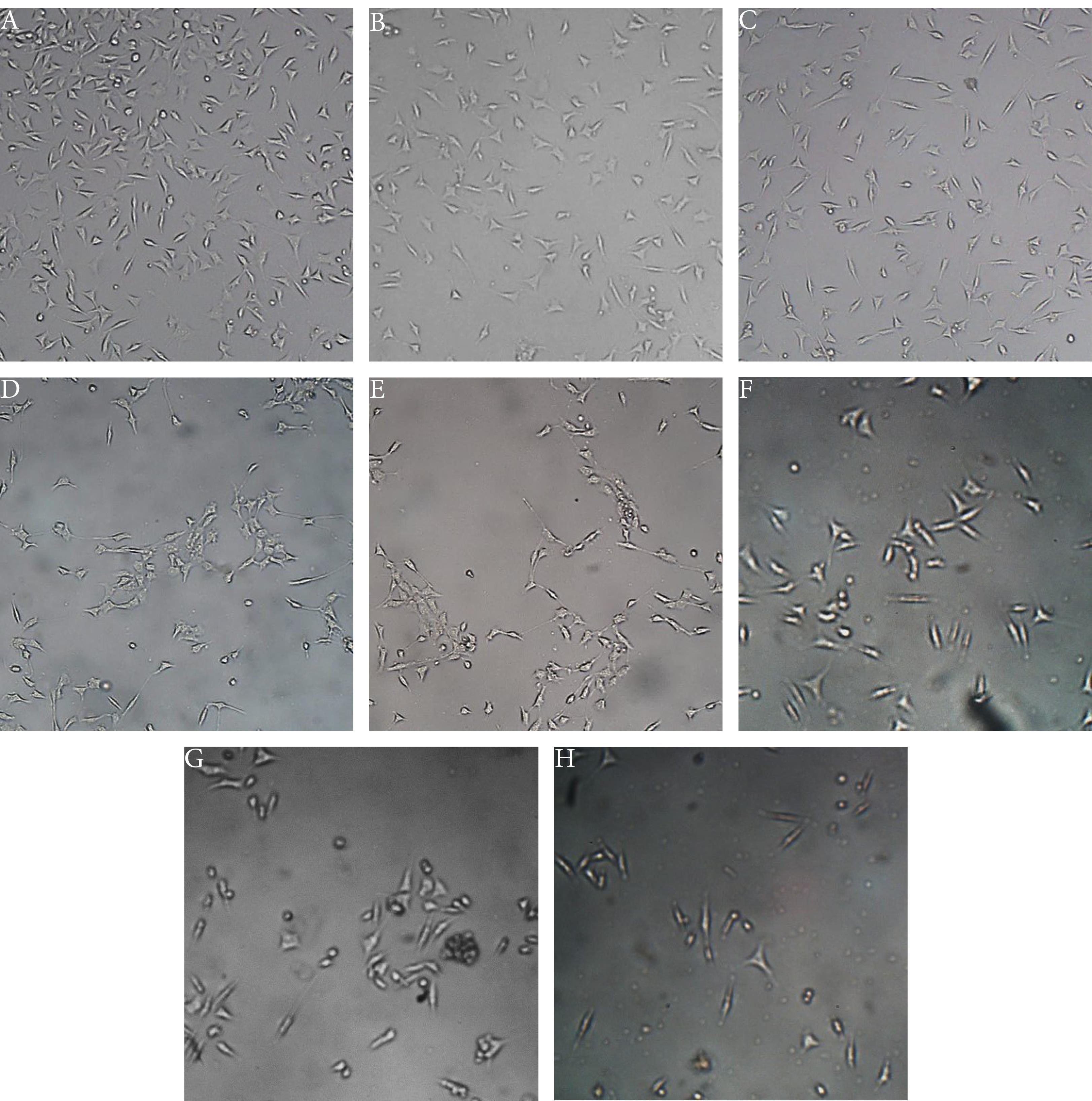
Figure 1.
Representative photomicrographs of the cellular morphology of MDA-MB-231 cells during the treatment period. The images illustrate the effect of different treatment conditions on cell morphology. (A) Untreated control: MDA-MB-231 cells without any treatment, serving as the baseline for comparison. (B) Co-culture with Jurkat cells: MDA-MB-231 cells cultured in the presence of Jurkat cells, without any additional treatment. (C, E, G) Treatment with 15, 30, and 60 μM LipDau: MDA-MB-231 cells treated with increasing concentrations (15, 30, and 60 μM) of LipDau, demonstrating dose-dependent effects. (D, F, H) Co-culture with 15, 30, and 60 μM LipDau-stimulated Jurkat cells: MDA-MB-231 cells co-cultured with Jurkat cells that have been stimulated with 15, 30, and 60 μM LipDau, respectively. The images show variations in cell morphology under different treatment conditions, with a scale bar of 200 μm indicating the magnification level.
.
Representative photomicrographs of the cellular morphology of MDA-MB-231 cells during the treatment period. The images illustrate the effect of different treatment conditions on cell morphology. (A) Untreated control: MDA-MB-231 cells without any treatment, serving as the baseline for comparison. (B) Co-culture with Jurkat cells: MDA-MB-231 cells cultured in the presence of Jurkat cells, without any additional treatment. (C, E, G) Treatment with 15, 30, and 60 μM LipDau: MDA-MB-231 cells treated with increasing concentrations (15, 30, and 60 μM) of LipDau, demonstrating dose-dependent effects. (D, F, H) Co-culture with 15, 30, and 60 μM LipDau-stimulated Jurkat cells: MDA-MB-231 cells co-cultured with Jurkat cells that have been stimulated with 15, 30, and 60 μM LipDau, respectively. The images show variations in cell morphology under different treatment conditions, with a scale bar of 200 μm indicating the magnification level.
Cell viability
The results from the MTT assay demonstrate that treatment with varying concentrations of LipDau significantly decreases the viability of MDA-MB-231 cells in a dose-dependent manner (Figure 2). Specifically, at concentrations of 15, 30, and 60 μM, LipDau reduced the viability of MDA-MB-231 cells by 21.1%, 49.9%, and 75.1%, respectively, all in comparison to the control group, with statistical significance indicated by P < 0.001. This clearly establishes LipDau as an effective agent for reducing cell viability in breast cancer cells. In contrast, co-culturing MDA-MB-231 cells with Jurkat cells resulted in only a modest decline in cell viability of 7.9% compared to control conditions (P > 0.05), suggesting that Jurkat cells alone do not have a significant inhibitory effect on the viability of MDA-MB-231 cells. Interestingly, the viability of Jurkat cells treated with 15, 30, and 60 μM LipDau also showed marked decreases of 34.6%, 61.5%, and 84.4%, respectively (P < 0.0001). This indicates that LipDau is effective not only against MDA-MB-231 cells but also significantly impacts Jurkat cell viability. When examining the effects of MDA-MB-231 cells cultured with Jurkat cells that were stimulated by LipDau, the results indicated a decline in cell viability of MDA-MB-231 cells at rates of 22.5% (P = 0.02), 11.6% (P = 0.04), and 9.3% (P = 0.15), showing reduced viability compared to the control. The decrease in viability appears to be less pronounced than the direct treatment with LipDau alone, but it indicates that the stimulated Jurkat cells still exert some effect on MDA-MB-231 cell viability. Overall, these findings suggest that while LipDau is highly effective in directly reducing MDA-MB-231 cell viability, co-culture with stimulated Jurkat cells also contributes to cell death but to a lesser extent. This paves the way for further investigation into potential combinatory effects of LipDau and Jurkat cell stimulation in enhancing the therapeutic impact on breast cancer cells.

Figure 2.
Analysis of the viability of MDA-MB-231 cells under various treatment conditions. (A) Bar graph showing the viability of MDA-MB-231 cells in the presence of different concentrations of LipDau (15, 30, 60 μM), Jurkat cells, and LipDau-stimulated Jurkat cells (15, 30, 60 μM). (B) Dose-response curve showing the effect of LipDau on MDA-MB-231 cells, with an IC50 value of 34.1 μM. (B) and (C) represent dose-response curve to determination of LipDau and stimulated Jurkat cells, respectively. Statistical significance is indicated by asterisks (*P < 0.05, ** P < 0.01, ***P < 0.001). This figure highlights the impact of LipDau and LipDau-stimulated Jurkat cells on the viability of MDA-MB-231 cells, emphasizing the dose-dependent effects and potential therapeutic implications.
.
Analysis of the viability of MDA-MB-231 cells under various treatment conditions. (A) Bar graph showing the viability of MDA-MB-231 cells in the presence of different concentrations of LipDau (15, 30, 60 μM), Jurkat cells, and LipDau-stimulated Jurkat cells (15, 30, 60 μM). (B) Dose-response curve showing the effect of LipDau on MDA-MB-231 cells, with an IC50 value of 34.1 μM. (B) and (C) represent dose-response curve to determination of LipDau and stimulated Jurkat cells, respectively. Statistical significance is indicated by asterisks (*P < 0.05, ** P < 0.01, ***P < 0.001). This figure highlights the impact of LipDau and LipDau-stimulated Jurkat cells on the viability of MDA-MB-231 cells, emphasizing the dose-dependent effects and potential therapeutic implications.
The results provide important insights into the efficacy of LipDau in targeting both MDA-MB-231 cells and Jurkat cells through the determination of IC50 values. In this study, the determined IC50 value for the direct effects of LipDau on MDA-MB-231 cells was found to be 31.1 μM. In contrast, the IC50 value for stimulated Jurkat cells was calculated to be 21.5 μM, which is lower than the IC50 for LipDau alone. This finding suggests that Jurkat cells are more sensitive to LipDau stimulation, requiring a lower concentration to achieve a significant effect.
According to these results, LipDau appears to be more potent in stimulating Jurkat cells than in directly inhibiting MDA-MB-231 cell viability. This is significant as it indicates the potential for using lower concentrations of LipDau to activate Jurkat cells, enhancing their ability to induce apoptosis in MDA-MB-231 cells. Moreover, the observation that lower concentrations of LipDau can stimulate Jurkat cells effectively suggests a strategy where LipDau is used not just as a cytotoxic agent directly against MDA-MB-231 cells, but also to prime Jurkat cells. When these stimulated Jurkat cells are co-cultured with MDA-MB-231 cells, they may induce additional cell death by utilizing immune mechanisms, possibly through apoptosis.
Comparison of apoptosis induction
The result of apoptosis investigation (Figure 3A-D) indicated that in the control cell group, the majority of cells were alive (85.5% in Q4). The percentage of living cells was 85% in MDA-MB-231 cells co-cultured with Jurkat cells. However, cells treated with LipDau and co-cultured with stimulated Jurkat cells showed lower percentages of live cells: 35.9% and 31.5%, respectively. Figure 3D demonstrates the absence of cells under treatment condition, which is consistent with the significant cytotoxic effect observed in our experiments. This result was expected based on our preliminary dose-response trials. This reveals a decrease in the percentage of MDA-MB-231 live cells following treatment with LipDau and stimulated Jurkat cells.

Figure 3.
Distribution of MDA-MB-231 cell from different treatments (A, control; B, 31.1 µM LipDau; C, Jurkat cells; D, 21.5 µM LipDau stimulated Jurkat cells) in four quartline of flow cytometery results. (E) Summary and comparison of apoptosis between groups. Statistical significance is indicated by asterisks (***P < 0.001). While co-culture with Jurkat cells alone did not significantly affect apoptosis compared to the control, LipDau treatment led to a significant increase in apoptosis.
.
Distribution of MDA-MB-231 cell from different treatments (A, control; B, 31.1 µM LipDau; C, Jurkat cells; D, 21.5 µM LipDau stimulated Jurkat cells) in four quartline of flow cytometery results. (E) Summary and comparison of apoptosis between groups. Statistical significance is indicated by asterisks (***P < 0.001). While co-culture with Jurkat cells alone did not significantly affect apoptosis compared to the control, LipDau treatment led to a significant increase in apoptosis.
Figure 3E summarizes and compare the differences of apoptosis results from flow cytometry. This figure shows the average percentage of cells at the beginning and end of apoptosis, with the average of three repetitions for each group. The results indicate that co-culturing MDA-MB-231 cells with Jurkat cells alone does not significantly affect the rate of apoptosis, as there is no statistically significant difference in apoptotic cells compared to the control group (P > 0.05). This suggests that Jurkat cells, in their unstimulated form, do not enhance apoptosis in MDA-MB-231 cells. However, treatment of MDA-MB-231 cells with LipDau markedly increases apoptosis, with a significant 51.2% rise (P < 0.001). This suggests that LipDau has a strong pro-apoptotic effect on MDA-MB-231 cells, independent of any interaction with Jurkat cells. Additionally, the results reveal that when MDA-MB-231 cells are co-cultured with stimulated Jurkat cells, there is a further increase in apoptosis, reaching 55.6% (P < 0.001). This indicates that the stimulated Jurkat cells also possess an apoptotic effect on MDA-MB-231 cells. Crucially, the study found a significant 49% increase in apoptosis in MDA-MB-231 cells co-cultured with stimulated Jurkat cells when compared to those co-cultured with unstimulated Jurkat cells (P < 0.001). This highlights that stimulation of Jurkat cells enhances their capability to induce apoptosis in MDA-MB-231 cells. Overall, these results suggest that while Jurkat cells alone do not promote apoptosis, their stimulation via LipDau substantially boosts their apoptotic effect on MDA-MB-231 cells, indicating the potential of using such combinations in therapeutic strategies against breast cancer.
CTLA-4 and COX-1 expression
The expression levels of the CTLA-4 gene in MDA-MB-231 cells were significantly altered by co-culture and treatment conditions. Specifically, MDA-MB-231 cells co-cultured with Jurkat cells exhibited a 20.4% reduction in CTLA-4 expression compared to control cells, although this did not reach statistical significance (P > 0.05; Figure 4A). Treatment with LipDau at a concentration of 31.1 μM resulted in a substantial 64.9% decrease in CTLA-4 expression relative to control cells (P = 0.002). Furthermore, MDA-MB-231 cells co-cultured with stimulated Jurkat cells demonstrated a remarkable 77% reduction in CTLA-4 expression compared to control cells (P = 0.0009), indicating a strong effect from the stimulated Jurkat cells. These data suggest that co-culturing MDA-MB-231 cells with Jurkat cells or treating them with LipDau significantly reduces the expression of the CTLA-4 gene. The combination of co-culturing with LipDau-stimulated Jurkat cells results in the most substantial reduction in CTLA-4 gene expression.
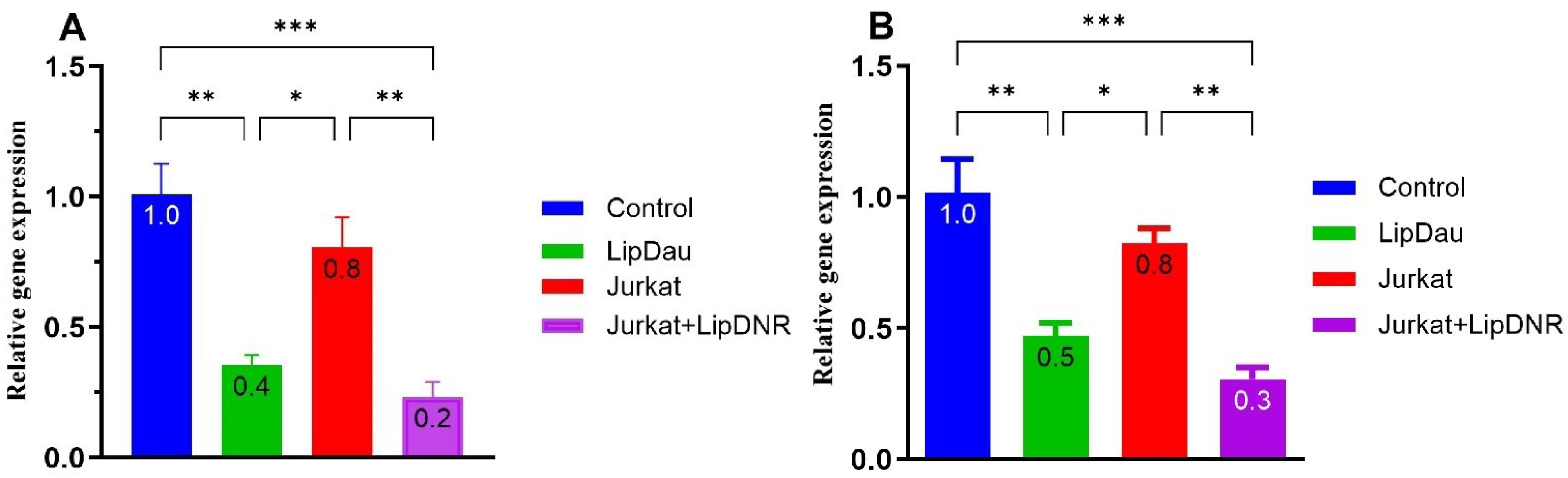
Figure 4.
Relative expression levels of CTLA-4 (A) and COX-1 (B) genes in various treatments of MDA-MB-231 cells: Control (untreated MDA-MB-231 cells); Jurkat (co-cultured with Jurkat cells); LipDau (treated with 31.1 μM LipDau); Jurkat-LipDau (co-cultured with 21.5 μM LipDau-stimulated Jurkat cells). LipDau treatment significantly reduces CTLA-4 and COX-1 gene expression in MDA-MB-231 cells. Co-culturing with LipDau-stimulated Jurkat cells further enhances this reduction, demonstrating LipDau’s potent effect. Statistical significance is indicated by asterisks (*P < 0.05, ** P < 0.01, *** P < 0.001).
.
Relative expression levels of CTLA-4 (A) and COX-1 (B) genes in various treatments of MDA-MB-231 cells: Control (untreated MDA-MB-231 cells); Jurkat (co-cultured with Jurkat cells); LipDau (treated with 31.1 μM LipDau); Jurkat-LipDau (co-cultured with 21.5 μM LipDau-stimulated Jurkat cells). LipDau treatment significantly reduces CTLA-4 and COX-1 gene expression in MDA-MB-231 cells. Co-culturing with LipDau-stimulated Jurkat cells further enhances this reduction, demonstrating LipDau’s potent effect. Statistical significance is indicated by asterisks (*P < 0.05, ** P < 0.01, *** P < 0.001).
In a parallel assessment, COX-1 gene expression showed similar patterns (Figure 4B). Co-culture with Jurkat cells led to a 21.6% decrease in COX-1 expression compared to control (P > 0.05). Cells treated with LipDau and co-cultured with stimulated Jurkat cells exhibited significant reductions of 57.2% (P < 0.001) and 72.1% (P < 0.001) in COX-1 expression compared to controls, respectively. Additionally, MDA-MB-231 cells co-cultured with stimulated Jurkat cells showed a 50.4% decrease in COX-1 expression compared to those co-cultured with unstimulated Jurkat cells (P < 0.01). These findings suggest that both LipDau treatment and co-culture with stimulated Jurkat cells significantly downregulate COX-1 gene expression in MDA-MB-231 cells. The most substantial reduction is observed when cells are co-cultured with stimulated Jurkat cells, highlighting the potential impact of Jurkat cell activation on COX-1 expression.
Discussion
Recent advancements in breast cancer research have prompted a re-evaluation of the immune system’s role in both disease progression and treatment response, particularly within the realm of immunotherapy.17 Immunotherapeutic strategies for breast cancer have evolved rapidly in recent years. Immune checkpoint inhibitors are being utilized to enhance T cell anti-tumor responses while countering immune evasion by tumor cells.1 However, breast cancer typically exhibits lower T cell infiltration and reduced mutational burdens compared to other malignancies, alongside substantial heterogeneity, which complicates treatment approaches.5
Anthracyclines, such as Dox and daunorubicin (Dau), possess immunomodulatory properties that can enhance the immune system’s ability to fight cancer.18 Our findings indicate that LipDau, when used to stimulate Jurkat (T lymphocyte) cells, results in a lower IC50 value compared to its direct action on MDA-MB-231 (metastatic breast cancer) cells. This suggests that LipDau is particularly effective in enhancing T cell activity against breast cancer. Utilizing stimulated Jurkat cells instead of direct LipDau treatment, akin to T cell therapies, may mitigate the adverse effects commonly associated with anthracycline therapy. This approach can promote immunogenic cell death, subsequently increasing the release of interleukins (ILs) and interferon-gamma (IFN-γ), while also fostering dendritic and T cell infiltration into tumors. Moreover, anthracyclines can be combined with immunotherapy to augment their therapeutic impact.19 Comparatively, similar studies have shown that the use of immune checkpoint inhibitors, such as nivolumab, loaded on gelatin nanoparticles (GNPs) also resulted in a lower IC50 value compared to free nivolumab solution. This indicates that enhancing effector cell activity through targeted delivery systems can significantly improve the efficacy of cancer treatments.20
While anthracyclines like Dox are effective in cancer treatment, they also induce cell death in both healthy and cancerous cells. Dox can initiate various forms of regulated cell death, including autophagy, ferroptosis, nephroptosis, pyroptosis, and apoptosis, depending on the cell type and drug concentration.21 In our study, we found that apoptosis induction is the predominant mechanism of cell death resulting from LipDau treatment in MDA-MB-231 cells. Unlike Jurkat cells, which do not contribute to cell death in MDA-MB-231 cells, stimulation of Jurkat cells with LipDau leads to MDA-MB-231 cell death primarily through apoptosis. Comparatively, previous studies have shown similar mechanisms of apoptosis induction in MDA-MB-231 cells. For instance, Jo et al22 demonstrated that Oligonol, a catechin-rich biotechnology product, induced apoptosis in MDA-MB-231 cells through the regulation of Bcl-2 family proteins and the MEK/ERK signaling pathway Additionally, Khaw-On et al23 reported that goniothalamin (GTN) induced apoptosis in MDA-MB-231 cells via intrinsic and extrinsic pathways, including ER stress and mitochondrial dysfunction. Wang et al24 suggested that Dox-induced apoptosis is p53-independent in normal cells but p53-dependent in cancer cells. The concentration of Dox can dictate the specific apoptotic pathway activated; at lower concentrations, apoptosis is triggered via AMPK and p53 activation, resulting in immediate loss of membrane integrity, while higher concentrations activate NF-kB, p38 MAPK, and caspases, leading to delayed loss of membrane integrity.25
Cancer cells often evade antitumor T cell responses by producing immune inhibitory molecules such as CTLA-4. Although research in this area is limited, existing studies suggest associations between CTLA-4 expression in the tumor microenvironment and poor clinical outcomes in breast cancer. Furthermore, CTLA-4 expression in breast cancer holds potential as a prognostic marker and a therapeutic target in evolving immunotherapy strategies.26 Our findings show that LipDau reduces CTLA-4 expression in MDA-MB-231 cells, and additionally, the stimulation of Jurkat cells with LipDau significantly decreases CTLA-4 expression in co-cultured MDA-MB-231 cells. These findings align with previous research indicating the role of immune modulation in cancer therapy. For instance, Grubczak et al27 reported that the suppression of CTLA-4 expression in tumor cells can enhance the efficacy of immune checkpoint inhibitors, thereby improving the anti-tumor response Furthermore, our results are consistent with studies on the use of various agents to modulate immune responses in cancer therapy. Rios-Doria et al demonstrated that Dox and Doxil synergize with anticancer immunotherapies, such as anti-PD-1 and CTLA-4 monoclonal antibodies, to improve tumor responses in mouse models. These anthracyclines elicit an immune response in T-cells against tumors, promoting the infiltration of CD8 + T cells and increasing CD80 expression on dendritic cells.19 Additionally, Li et al28 found that the mAb B1C4 significantly raised CTLA-4 secretion levels in Jurkat cells within a co-culture system, enhancing the cytotoxic activity of Jurkat cells against HepG2 cells.
COX-1, typically associated with maintaining cellular and tissue homeostasis, may also play a role in cancer. Increased COX-1 expression has been observed in certain cancers, suggesting its potential involvement in tumorigenesis alongside PTGS2.12 In this study, we noted a decrease in COX-1 expression following LipDau treatment of MDA-MB-231 cells. Furthermore, co-culturing induced Jurkat cells with MDA-MB-231 cells significantly decreased COX-1 expression, while Jurkat cells alone showed negligible effects. This finding aligns with previous research indicating that COX-1 expression can be modulated by various treatments in cancer cells. For instance, a study by Díaz-Cruzet al29 demonstrated that COX inhibitors could suppress aromatase expression and activity in breast cancer cells, suggesting a potential mechanism for the observed decrease in COX-1 expression Elevated levels of COX-1 expression in breast cancer cells indicate its classification as an oncogenic component.30 Inhibition of COX-1 has also been associated with reduced breast cancer metastasis.31 The role of type 1 conventional dendritic cells (cDC1s) in anti-cancer immunity is crucial as they acquire antigens from tumor cells and activate cancer-specific CD8 + T cells.32-34 The activity of cDC1s within tumors can be influenced by the bioactive lipid prostaglandin E2 (PGE2), produced by COX-1 and PTGS2 enzymes.13 The COX-1 inhibitor, FR122047, has been shown to induce cell growth arrest and apoptosis in MCF-7 human breast cancer cells.35 Collectively, these results suggest that the stimulation of Jurkat cells with LipDau not only decreases COX-1 expression but also facilitates apoptosis in MDA-MB-231 cells.
Conclusion
Our study uncovered significant differences in the impact of Jurkat cells and stimulated Jurkat cells on MDA-MB-231 (metastatic breast cancer) cells. We found that LipDau-stimulated Jurkat cells elicited an anti-tumor response by the CTLA4 checkpoint molecule and suppressed the inflammatory response leading to tumor immune evasion by COX-1 in MDA-MB-231 cells. These responses resulted in apoptosis induction and suppression of invasion in metastatic breast cancer cells. Our study offered preliminary data suggesting that LipDau-induced Jurkat cells could be a potential tool in cancer immunotherapy, but more comprehensive studies are needed to support our findings. Further techniques and analyses are required to understand the exact reasons behind these results.
Competing Interests
The authors declare no conflict of interest.
References
- Hu Y, Li Y, Yao Z, Huang F, Cai H, Liu H. Immunotherapy: review of the existing evidence and challenges in breast cancer. Cancers (Basel) 2023; 15(3):563. doi: 10.3390/cancers15030563 [Crossref] [ Google Scholar]
- Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers (Basel) 2021; 13(17):4287. doi: 10.3390/cancers13174287 [Crossref] [ Google Scholar]
- García-Aranda M, Redondo M. Immunotherapy: a challenge of breast cancer treatment. Cancers (Basel) 2019; 11(12):1822. doi: 10.3390/cancers11121822 [Crossref] [ Google Scholar]
- Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020; 20(11):651-68. doi: 10.1038/s41577-020-0306-5 [Crossref] [ Google Scholar]
- Akkın S, Varan G, Bilensoy E. A review on cancer immunotherapy and applications of nanotechnology to chemoimmunotherapy of different cancers. Molecules 2021; 26(11):3382. doi: 10.3390/molecules26113382 [Crossref] [ Google Scholar]
- Quijano-Rubio A, Ulge UY, Walkey CD, Silva DA. The advent of de novo proteins for cancer immunotherapy. Curr Opin Chem Biol 2020; 56:119-28. doi: 10.1016/j.cbpa.2020.02.002 [Crossref] [ Google Scholar]
- Mattioli R, Ilari A, Colotti B, Mosca L, Fazi F, Colotti G. Doxorubicin and other anthracyclines in cancers: activity, chemoresistance and its overcoming. Mol Aspects Med 2023; 93:101205. doi: 10.1016/j.mam.2023.101205 [Crossref] [ Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8):711-23. doi: 10.1056/NEJMoa1003466 [Crossref] [ Google Scholar]
- Zhang H, Dutta P, Liu J, Sabri N, Song Y, Li WX. Tumour cell-intrinsic CTLA4 regulates PD-L1 expression in non-small cell lung cancer. J Cell Mol Med 2019; 23(1):535-42. doi: 10.1111/jcmm.13956 [Crossref] [ Google Scholar]
- Sun L, Chen L, Li H. Checkpoint-modulating immunotherapies in tumor treatment: targets, drugs, and mechanisms. Int Immunopharmacol 2019; 67:160-75. doi: 10.1016/j.intimp.2018.12.006 [Crossref] [ Google Scholar]
- Baksh K, Weber J. Immune checkpoint protein inhibition for cancer: preclinical justification for CTLA-4 and PD-1 blockade and new combinations. Semin Oncol 2015; 42(3):363-77. doi: 10.1053/j.seminoncol.2015.02.015 [Crossref] [ Google Scholar]
- Pannunzio A, Coluccia M. Cyclooxygenase-1 (COX-1) and COX-1 inhibitors in cancer: a review of oncology and medicinal chemistry literature. Pharmaceuticals (Basel) 2018; 11(4):101. doi: 10.3390/ph11040101 [Crossref] [ Google Scholar]
- Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 2018;172(5):1022-37.e14. doi: 10.1016/j.cell.2018.01.004.
- Oztatlici M, Özdemir AT, Oztatlici H, Kucukhuyuk S, Özgül Özdemir RB, Orhan H. Immunomodulatory effects of MDA-MB-231-derived exosome mimetic nanovesicles on CD4 + T cell line. Eurasian J Med Oncol 2024; 8(1):40-8. doi: 10.14744/ejmo.2024.64067 [Crossref] [ Google Scholar]
- Alaa Hadi-Al-Ward N, Ebrahimi M, Zare Karizi S. Investigation of the cytotoxic and antiproliferative effects of liposomal daunorubicin on human colorectal cancer (HCT116) cell line. Iran J Pharm Res 2024; 23(1):e144287. doi: 10.5812/ijpr-144287 [Crossref] [ Google Scholar]
- Chen W, Li L, Zhang X, Liang Y, Pu Z, Wang L. Curcumin: a calixarene derivative micelle potentiates anti-breast cancer stem cells effects in xenografted, triple-negative breast cancer mouse models. Drug Deliv 2017; 24(1):1470-81. doi: 10.1080/10717544.2017.1381198 [Crossref] [ Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022; 72(1):7-33. doi: 10.3322/caac.21708 [Crossref] [ Google Scholar]
- Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med 2011; 208(3):491-503. doi: 10.1084/jem.20100269 [Crossref] [ Google Scholar]
- Rios-Doria J, Durham N, Wetzel L, Rothstein R, Chesebrough J, Holoweckyj N. Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models. Neoplasia 2015; 17(8):661-70. doi: 10.1016/j.neo.2015.08.004 [Crossref] [ Google Scholar]
- Ali DS, Gad HA, Hathout RM. Enhancing effector Jurkat cell activity and increasing cytotoxicity against A549 cells using nivolumab as an anti-PD-1 agent loaded on gelatin nanoparticles. Gels 2024; 10(6):352. doi: 10.3390/gels10060352 [Crossref] [ Google Scholar]
- Christidi E, Brunham LR. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis 2021; 12(4):339. doi: 10.1038/s41419-021-03614-x [Crossref] [ Google Scholar]
- Jo EH, Lee SJ, Ahn NS, Park JS, Hwang JW, Kim SH. Induction of apoptosis in MCF-7 and MDA-MB-231 breast cancer cells by Oligonol is mediated by Bcl-2 family regulation and MEK/ERK signaling. Eur J Cancer Prev 2007; 16(4):342-7. doi: 10.1097/01.cej.0000236247.86360.db [Crossref] [ Google Scholar]
- Khaw-On P, Pompimon W, Banjerdpongchai R. Apoptosis induction via ATM phosphorylation, cell cycle arrest, and ER stress by goniothalamin and chemodrugs combined effects on breast cancer-derived MDA-MB-231 cells. Biomed Res Int 2018; 2018:7049053. doi: 10.1155/2018/7049053 [Crossref] [ Google Scholar]
- Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms intermediacy of H2O2- and p53-dependent pathways. J Biol Chem 2004; 279(24):25535-43. doi: 10.1074/jbc.M400944200 [Crossref] [ Google Scholar]
- Eom YW, Kim MA, Park SS, Goo MJ, Kwon HJ, Sohn S. Two distinct modes of cell death induced by doxorubicin: apoptosis and cell death through mitotic catastrophe accompanied by senescence-like phenotype. Oncogene 2005; 24(30):4765-77. doi: 10.1038/sj.onc.1208627 [Crossref] [ Google Scholar]
- Kern R, Panis C. CTLA-4 expression and its clinical significance in breast cancer. Arch Immunol Ther Exp (Warsz) 2021; 69(1):16. doi: 10.1007/s00005-021-00618-5 [Crossref] [ Google Scholar]
- Grubczak K, Kretowska-Grunwald A, Groth D, Poplawska I, Eljaszewicz A, Bolkun L. Differential response of MDA-MB-231 and MCF-7 breast cancer cells to in vitro inhibition with CTLA-4 and PD-1 through cancer-immune cells modified interactions. Cells 2021; 10(8):2044. doi: 10.3390/cells10082044 [Crossref] [ Google Scholar]
- Li Z, Li B, Li L, Wang G, Li Y, Fu R. The immunostimulative effect and mechanisms of a novel mouse anti-human PD-1 monoclonal antibody on Jurkat lymphocytic cells cocultured with hepatoma cells. Onco Targets Ther 2020; 13:12225-41. doi: 10.2147/ott.S281397 [Crossref] [ Google Scholar]
- Díaz-Cruz ES, Shapiro CL, Brueggemeier RW. Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab 2005; 90(5):2563-70. doi: 10.1210/jc.2004-2029 [Crossref] [ Google Scholar]
- Gatto F, Ferreira R, Nielsen J. Pan-cancer analysis of the metabolic reaction network. Metab Eng 2020; 57:51-62. doi: 10.1016/j.ymben.2019.09.006 [Crossref] [ Google Scholar]
- Ni L, Zhu X, Zhao Q, Shen Y, Tao L, Zhang J. Dihydroartemisinin, a potential PTGS1 inhibitor, potentiated cisplatin-induced cell death in non-small cell lung cancer through activating ROS-mediated multiple signaling pathways. Neoplasia 2024; 51:100991. doi: 10.1016/j.neo.2024.100991 [Crossref] [ Google Scholar]
- Böttcher JP, Reis e Sousa C. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer 2018; 4(11):784-92. doi: 10.1016/j.trecan.2018.09.001 [Crossref] [ Google Scholar]
- Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S. Expansion and activation of CD103 + dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 2016; 44(4):924-38. doi: 10.1016/j.immuni.2016.03.012 [Crossref] [ Google Scholar]
- Hubert M, Gobbini E, Couillault C, Manh TV, Doffin AC, Berthet J. IFN-III is selectively produced by cDC1 and predicts good clinical outcome in breast cancer. Sci Immunol 2020; 5(46):eaav3942. doi: 10.1126/sciimmunol.aav3942 [Crossref] [ Google Scholar]
- Jeong HS, Kim JH, Choi HY, Lee ER, Cho SG. Induction of cell growth arrest and apoptotic cell death in human breast cancer MCF-7 cells by the COX-1 inhibitor FR122047. Oncol Rep 2010; 24(2):351-6. doi: 10.3892/or_00000866 [Crossref] [ Google Scholar]